INTRODUCTION
Sj├Čgren's syndrome (SS) is a chronic, progressive autoimmune disease that mainly affects exocrine glands including the salivary and lacrimal glands1). Functional impairment of these glands by focal lymphocyte infiltration diminishes the production of tears and saliva and produces the clinical findings of persistent dry mouth and eyes. In addition to glandular manifestations, some patients with SS have a disorder that is complicated by multiple organ involvement; extra-glandular or systemic clinical features such as: arthritis, fatigue, lymphoma, and vasculitis may be present2). Antinuclear antibody (ANA) and rheumatoid factor (RF) are frequently detected in serologic tests of these patients in the course of the diagnosis of SS. Antibodies directed to extractable nuclear antigens, such as anti-Ro or anti-La, are known to be more specific for a diagnosis of SS3).
SS may occur as primary SS, which develops alone, or it can be associated with a variety of systemic autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, or primary biliary cirrhosis4-6). Few cases of SS associated with seronegative spondyloarthropathy (SpA), such as ankylosing spondylitis, reactive arthritis (ReA), psoriatic arthritis, and undifferentiated SpA, have been reported until recently7-9). However, most of the overlapping features have occurred in undifferentiated SpA and SS, whereas SS in patients with ReA is rarely reported in the literature. We report a case of SS and concurrent Chlamydia-induced ReA; this is the first report in the Korean population.
CASE REPORT
A 40-year-old man presented with a history of swelling and pain of both ankle joints and plantar surfaces, and inflammatory back pain for 5 years. He also had dry mouth and eyes, and fatigue. The patient had a medical history of urethritis with a recurrent dysuria and pyuria, which was intermittently treated with systemic antibiotics.
A physical examination revealed significant swelling of both ankle joints and severe tenderness around the bony insertion portions of the Achilles tendon. Tenderness was also noted in the: iliac crest (bilaterally), tibial tuberosities, and calcaneus. Bilateral keratoconjunctivitis sicca was confirmed by an ophthalmologic examination which included the Schimer's test (3 mm left eye and 2 mm right eye). However characteristic manifestations of ReA including keratoderma blennorrhagica, circinate balanitis, and painless oral ulcer were absent.
Laboratory analyses on admission demonstrated a C-reactive protein of 2.0 mg/dL (normal: < 0.3 mg/dL) and a erythrocyte sedimentation rate of 36 mm/hr (normal: 0-20 mm/hr). Complete blood count and blood biochemistry produced normal findings. Routine analysis and culture of urine showed pyruia (WBC 10-19/high power field) without identification of any bacterial organisms. HLA-B27 was positive. ANA was detected at a titer of 1:320. RF and anti-Ro antibody were elevated to 56 IU/mL (normal: < 15 IU/mL) and 460 AAU (normal: < 150 AAU), respectively. However, anti-neutrophil cytoplasmic antibody, double-strand DNA antibody, anti-Sm antibody, anti-RNP antibody, and anti-La antibody were all negative. IgG titer for Chlamydia trochomonas was 4.0 (normal: <0.9); this value is almost four fold elevated compared to the cut-off value using the ELISA method.
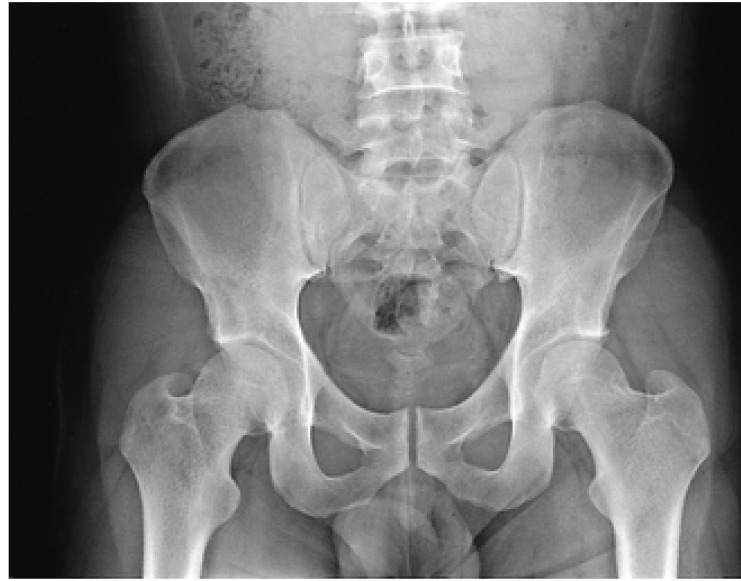
The radiological finding of the sacroiliac joints revealed mild marginal irregularity and subchondral sclerosis at the left joint surface (Figure 1). In addition to periostitis, soft tissue thickening, and subchondral sclerosis along the inferior and posterior aspects of the calcaneus, bony erosion was also present at the bony insertion site of the Achilles tendon (Figure 2). A whole body bone scan using Tc99m-MDP showed more increased uptake at the left sacroiliac joint compared to the right joint; in addition, increased uptake of the left foot compared to the right was observed (Figure 3). A reduced excretion or abnormal uptake of the salivary glands was not noted in the evaluation by salivary scan.
On the basis of: inflammatory back pain, asymmetrical sacroiliitis, evidence of urethritis by Chlamydia infection, recurrent pyuria, peripheral arthritis dominant in lower extremities, and enthesitis of Achilles tendon with radiological changes, a diagnosis of ReA combined with SS was assigned to the patient. The criteria were fulfilled for the diagnosis of ReA based ib the European Spondylarthropathy Study Group (ESSG) and SS of European-American Consensus Group, respectively10, 11). For the enthesitis and arthritis, we administered low dose corticosteroid (prednisolone 5 mg/day), sulfasalazine (1,000 mg bid/day), and non-inflammatory anti-inflammatory drugs (NSAIDs). Intra-articular injection or intra-lesional infiltration of steroid was also performed for swollen joints and tender lesions of enthesopathy. Only 1 gram of oral azithromycine for urethritis was needed. Dry eyes were controlled by replacement with an artificial tear solution. The use of pilocarpine for dry mouth was withdrawn because of an erosive gastric lesion confirmed by endoscopy. After discharge from the hospital, inflammatory back pain, swollen joints, and enthesitis subsided. Laboratory findings indicating disease activity were also gradually improved. Pyuria resolved on repeat urine analysis.
DISCUSSION
There are a few reports in the literature on concurrent SpA and SS7-9). The clinical characteristics of SpA typically do not include the sicca symptoms of SS. There are overlapping clinical and laboratory features that occur in SpA (particularly AS or undifferentiated SpA) and SS. The case of Chlamydia-induced ReA and SS may be the first report identified in the Korean population.
Central to the pathogenesis of SS is a chronic stimulation of the humoral or cellular immune system; this includes B and T lymphocytes. Circulating autoantibodies and hypergammaglobulinemia are expressed by B cell hyperreactivity12). Organspecific autoantibodies for cellular antigens of the salivary gland are noted in patients with SS, whereas non-organ-specific autoantibodies are noted in approximately 60% of SS patients. These nonspecific autoantibodies include RF, ANA, and antibodies to the small RNA-protein complexes Ro/SS-A and La/SS-B; these are the best available autoantibodies for the diagnosis of SS. These autoantibodies may be related to functional impairment of exocrine glands by the destruction of glandular tissue13). Our patient presented with the chronic, persistent sicca symptoms of dry mouth and dry eyes, showed a positive result to the Schimer's test, and showed a specific autoantibody to the Ro/SS-A antigen. Our patient was diagnosed with SS without histopathologic investigation because the findings were compatible with the recently modified criteria for SS according to the European-American Consensus Group11).
ReA is a sterile inflammatory arthritis which may be triggered by a variety of urogenital or enteropathic infectious agents in the genetically susceptible host14). Causative organisms identified in association with ReA include the following species: Chlamydia, Ureaplasma, Shigella, Salmonella, Yersinia, and Campylobacter15). A well-known genetic predisposing factor is the HLA-B27 type, which has been shown to be strongly related to the occurrence of a group of inflammatory arthritis conditions known as SpA; although its specific role in these disorders has not been clearly determined16). ReA is typically an acute, asymmetric, and oligoarticular peripheral arthritis; the joints affected are preferentially the joints of the lower extremities. ReA is frequently associated with one or more characteristic extra-articular manifestations such as conjunctivitis, Achilles tendonitis, plantar fasciitis, dactylitis (sausage digits), urethritis, circinate balnitis, and, rarely, carditis12, 17). A positive history of recurrent urethritis and antibodies against Chlamydia was observed in this case. These extra-articular manifestations are necessary to support the diagnosis of reactive arthritis. Thus, this case also fulfilled the criteria for SpA according to the ESSG10).
RF and ANA are frequently detected in patients with SS, but these autoantibodies are not considered to be a typical feature of SpA. However, the frequency of reports of concurrent SS and SpA have recently increased in the published literature, although this association has not been formally accepted.7-9) Previous studies have examined the association between the development of SS and SpA; results have shown an increased prevalence of SS in SpA with positive ANA tests; this suggests that ANA may be a risk factor for the development of SS in SpA8, 9).
Prior studies have demonstrated that the prevalence of ANA in SpA is higher in patients with AS and psoriatic arthritis18, 19). Recent studies have also shown that ANA is increased in SpA patients when compared to controls; however, the prevalence was reported to be lower in RA patients8, 9). Although ANA is common in SpA patients, the specific role of ANA in the pathogenesis of SpA has not been elucidated.
The use of anti-inflammatory agents, including non-steroidal anti-inflammatory drugs (NSAIDs), is the cornerstone of treatment in the management of ReA.14) Corticosteroids have also proven to be clinically valuable. In the case of enthesis and persistent peripheral synovitis, intra-articular or intra-lesional corticosteroids are very effective. The addition of sulfasalazine or methotrexate to suppress disease activity may be needed in patients who are refractory or cannot tolerate NSAIDs. Long -term use of antibiotics has not been shown to be effective in controlling disease activity; although prompt treatment with doxycycline for Chlamydia infections reduced the risk of the development of ReA in one study20). We chose to use azithromycin, instead of tetracycline, to control urethritis because the former agent is simple, effective and well tolerated when compared to the latter. Recently, monoclonal antibodies or receptor antagonists of tumor necrosis factor (TNF) such as infliximab and etanercept have been used in the treatment of ReA and SS21, 22). If a patient proves to be refractory or cannot tolerate combined therapy including NSAIDs, sulfasalazine, and methotrexate, consideration of new biologic agents will follow. However, the value of these biologic agents for the treatment of these diseases remains to be determined; there is the possibility that reactivation-triggering bacteria may exacerbate disease symptoms after the administration of an anti-TNF agent. For the treatment of SS, the therapeutic effectiveness of TNF blocking agents is still undetermined.
In conclusion, we described a rare case that presented with overlapping clinical features of Chlamydia-induced ReA and SS. Further study is needed to determine the nature of this apparent association between SS and SpA.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





