 |
 |
| Korean J Intern Med > Volume 21(3); 2006 > Article |
|
Abstract
The Mucosa-associated lymphoid tissue (MALT) lymphoma, which was first described in 1983, is known to be caused by chronic Helicobacter pylori (HP) infection, which triggers lymphoid infiltration and formation of organized lymphoid tissue. In approximately two thirds of cases of MALT, the lymphoma has been observed to regress after treatment of H. pylori infection; this provides strong evidence of a causative role of HP in the etiology of MALT. We report a case of a 67-year-old female patient with a high-grade MALT lymphoma of the liver; this occurred six years after complete remission of a low-grade gastric MALT lymphoma and after complete eradication of H. pylori. there was no recurrence of the previous low-grade gastric MALT lymphoma. Based on radiological and pathologic findings, the high-grade MALT was considered to result from transformation of the low-grade gastric MALT lymphoma.
The stomach is one of the most common organs where MALT lymphoma develops. It is well established, that more than 90% of low-grade MALT lymphomas are associated with H. pylori infection1). Indeed, the eradication of H. pylori infection has been associated with the regression of gastric MALT lymphoma in 70% to 80% of patients2, 3). Additional factors contribute to the development of MALT lymphoma such as: environmental, microbial, immune and genetic factors. Transformation of MALT lymphoma has been described; there is genetic evidence for a clonal link between low- and high-grade components4). Here, we report a case of high-grade hepatic MALT lymphoma that occurred six years after achieving complete remission of a low-grade gastric MALT lymphoma with H. pylori eradication therapy. There was no recurrence of the low-grade gastric MALT lymphoma itself. Although we were unable to obtain cytogenetic evidence, the radiological and pathologic findings suggested that the low-grade MALT was transformed to a high-grade hepatic MALT lymphoma.
A 67-year-old woman visited our hospital with complaints of abdominal discomfort, indigestion and constipation for five days. Physical examination and laboratory findings were not specific, and her performance was excellent. Gastroscopic examination showed an ulcero-infiltrative lesion at the greater curvature side of the antrum (Figure 1). Histological examination revealed mucosal ulceration with diffuse infiltration of lymphoid cells. Diffuse and nodular aggregates of lymphoid cells were composed of monotonous small to medium-sized lymphocytes with round to cleaved nuclei and clear cytoplasm. In some areas, relatively large atypical lymphoid cells were also present in less than 10% of tumor cells. There were a few foci of lymphoepithelial lesions (Figure 2). Immunohistochemical stains were positive for CD20 and CD79a, but negative for CD45RO, CD3, CD5, CD23, Bcl-2 and Cyclin D1. H. pylori infection was detected using the rapid urease test and histology. An abdominal CT scan revealed focal wall thickening on the greater curvature side of antrum without any lymph node enlargement. Unfortunately, a bone marrow biopsy was not performed, thus staging work-up of the lymphoma was not fully performed. The patient was diagnosed with a low-grade gastric MALT lymphoma and treated for H. pylori infection for 14 days with omeprazole 20 mg bid, clarithromycin 500 mg bid and amoxicillin 1,000 mg bid. Follow-up gastroscopic examinations, one and three months after H. pylori eradication, showed marked improvement of gastric lesions. H. pylori infection was absent by rapid urease testing and histology, but MALT lymphoma was still present on histology evaluation. Thereafter the patient was lost to follow-up.
Four years later, the patient visited our hospital again with complaints of indigestion and constipation. Gastroscopy showed diffuse erythematous changes of the antral mucosa with an irregular surface at the site of the previous MALT lymphoma that appeared to be a scar. Gastric mucosal biopsy showed well preserved foveolar epithelium, and sparsely scattered inflammatory cells in the lamina propria devoid of residual lymphoma. H. pylori infection was not found using the rapid urease test and by histology. Abdominal CT scan showed neither gastric wall thickening nor lymph node enlargement (Figure 3). These findings were interpreted to represent complete remission of the gastric MALT lymphoma. The patient was then again lost to follow-up.
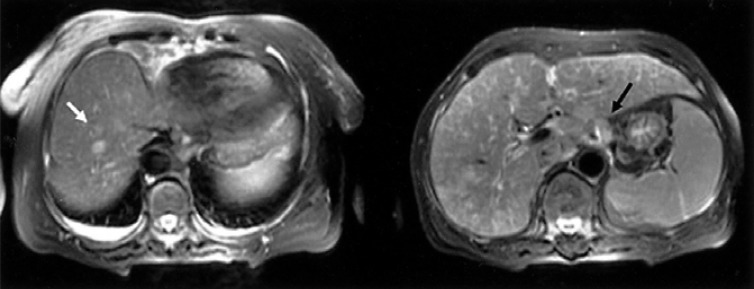
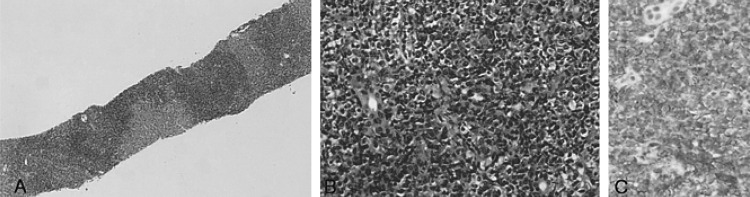
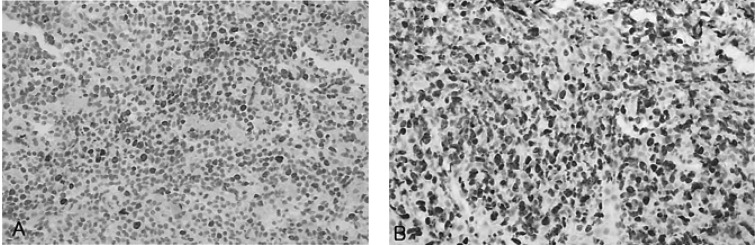
About two years later, the patient returned to our hospital with complaints of general weakness and epigastric discomfort. Blood tests on admission showed a hematocrit of 36.1%, a white blood cell count of 4,200/mm3, a platelet count of 77,000/mm3, a protein of 4.6 g/dL, an albumin of 2.4 g/dL, a total bilirubin of 3.0 mg/dL (0.2-1.0 mg/dL), a direct bilirubin of 2.5 mg/dL (0.0-0.2 mg/dL), an alkaline phosphatase of 588 U/L (32-104 U/L), an alanine aminotransferase of 196 U/L (10-36 U/L), an aspartate aminotransferase of 357 U/L (10-30 U/L), a lactate dehydrogenase of 1,871 U/L (211-423 U/L), and a prothrombin time of 11.8 sec (10-14 sec). Gastroscopic examination showed several round superficial ulcerations and diffuse mucosal erythema at the antrum. Histological examination revealed only chronic inflammation and intestinal metaplasia with the absence of H. pylori infection and MALT lymphoma (Figure 4). Abdominal sonography and liver MRI showed multiple hepatic nodules with multiple celiac and para-aortic lymphadenopathy (Figure 5). Ultrasonography-guided percutaneous core needle biopsy was performed on the hepatic nodules; histological examination revealed a patchy infiltration, of atypical lymphoid cells, replacing the liver parenchyma. There were conspicuous medium to large lymphoid cells admixed with monocytoid cells. The composition of the large lymphoid cells, found in the liver biopsy, was significantly increased when compared to the previous gastric MALT lymphoma. Immunohistochemical stains were positive for CD20, but negative for: CD45RO, CD3, CD5, CD23, Bcl-2 and Cyclin D1 (Figure 6). Immunohistochemical stains for Ki-67 were performed both on the biopsy from the previous low-grade gastric MALT lymphoma and the liver core biopsy. The Ki-67 labeling index, from the liver biopsy sample, was much higher (70%) than in the previous gastric biopsy (20%) (Figure 7). These pathological findings suggested a hepatic MALT lymphoma that represented a transformation from the previously treated low-grade gastric MALT lymphoma to a high-grade MALT lymphoma. The patient, and her family, refused further treatment and her clinical status deteriorated rapidly; she died from sepsis one month later.
The MALT lymphoma was first described as a distinct pathologic entity in 1983.5) In 1988, it became apparent that the gastric MALT lymphoma is caused by chronic H. pylori infection; this triggers lymphoid infiltration and formation of organized lymphoid tissue6, 7). Perhaps the strongest evidence is that in approximately two thirds of cases, the lymphoma regresses after treatment of the H. pylori infection2, 3). It is assumed that the initial proliferation of lymphoma cells occurs in an antigen-dependent fashion; this explains the tendency for these lymphomas to remain anatomically localized and to respond to antibacterial therapy. Given that most patients with H. pylori gastritis do not develop MALT lymphoma, additional events such as environmental, microbial, immune and genetic factors must play an important role8). Our patient was positive for H. pylori infection; the initial original gastric MALT lymphoma completely disappeared after eradication of H. pylori infection. However, the occurrence of transformation to a high-grade lymphoma without evidence of H. pylori re-infection strongly suggests that some other pathological mechanisms, besides H. pylori infection, played a role in disease progression.
The understanding of the biology of the gastric MALT lymphoma has improved over the past few years. There is a common structural cytogenetic abnormality associated with this disorder, t(11;18)(q21;q21), detected in 21% to 60% of cases8-11). This translocation acts to fuse the inhibitors of apoptosis (IAP) family API2 with the novel gene MALT1. The characterization of another, albeit less frequent recurrent translocation, t(1;14)(p22;q23) has led to the identification of Bcl1012, 13). Both translocations are known to result in activation of the central transcription factor NF-╬║B14). NF-╬║B induction appears to drive antigen independent growth of the lymphoma cells, promoting disease dissemination and unresponsiveness to H. pylori eradication therapy8).
Transformation of MALT lymphoma has been described, and there is a genetic evidence for a clonal link between low- and high-grade components4). Deletion of the p16, mutations of the p53 gene, translocation breakpoint involved in t(6;14)(p21;q32), and Bcl-6 have been described to be associated with transformation of the MALT lymphoma15-17). Moreover, numerous investigators have sequenced immunoglobulin heavy chain (IgH) genes, and have found frequent somatic mutations that can be ongoing18). Somatic hypermutations, together with isotype switch recombination, contributes to B-cell maturation. Unfortunately, we could not perform cytogenetic evaluations or IgH gene rearrangement analysis in our patient. However, in the pathological findings, the composition of the large lymphoid cells and the expression of the proliferation marker Ki-67 were significantly increased in the hepatic nodules compared to the previous gastric MALT lymphoma; these results suggested that the hepatic nodules represented a transformation to a higher grade malignant lymphoma from the prior low-grade gastric MALT lymphoma. In addition, the occurrence of high-grade transformation, even after complete remission of the original gastric lesion, its aggressive nature may suggest that our patient had one or more underlying cytogenetic abnormalities. However, the possibility that the hepatic MALT lymphoma might be a newly developed and separate lesion, with no relationship to the initial low-grade gastric MALT lymphoma, can not be completely excluded.
In summary, we report a case of high-grade hepatic MALT lymphoma that occurred six years after achieving complete remission of a low-grade gastric MALT lymphoma. Although we did not perform cytogenetic evaluation, the hepatic MALT lymphoma was considered to represent a transformation from the gastric MALT lymphoma on the basis of radiological and pathological findings. In addition, these findings suggest that the determination of a complete cure for MALT lymphoma based on gastroscopic and histological findings might not be adequate. Regular follow-up, even after complete remission of the original lesion, is mandatory, and cytogenetic studies should be considered in certain cases if available.
References
1. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991;338:1175ŌĆō1176PMID : 1682595.


2. Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosaassociated lymphoid tissue type after cure of Helicobacter pylori infection. Lancet 1995;345:1591ŌĆō1594PMID : 7783535.


3. Roggero E, Zucca E, Pinotti G, Pascarella A, Capella C, Savio A, Pedrinis E, Paterlini A, Venco A, Cavalli F. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995;122:767ŌĆō769PMID : 7717599.


4. Peng H, Du M, Diss TC, Isaacson PG, Pan L. Genetic evidence for a clonal link between low and high-grade components in gastric MALT B-cell lymphoma. Histopathology 1997;30:425ŌĆō429PMID : 9181363.


5. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue: a distinct type of B-cell lymphoma. Cancer 1983;52:1410ŌĆō1416PMID : 6193858.


6. Wyatt JI, Rathbone BJ. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl 1988;142:44ŌĆō49PMID : 3166533.


7. Stolte M, Eidt S. Lymphoid follicles in antral mucosa: immune response to Campylobacter pylori? J Clin Pathol 1989;42:1269ŌĆō1271PMID : 2613920.



9. Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, Horsman DE. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997;8:979ŌĆō985PMID : 9402171.


10. Baens M, Maes B, Steyls A, Geboes K, Marynen P, de Wolf-Peeters C. The product of the t(11;18), an API2-MLT fusion, marks nearly half of gastric MALT type lymphomas without large cell proliferation. Am J Pathol 2000;156:1433ŌĆō1439PMID : 10751367.



11. Liu H, Ye H, Ruskone-Fourmestraux A, de Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wundisch T, Molina T, Taal BG, Elena S, Thomas T, Zinzani PL, Neubauer A, Stolte M, Hamoudi RA, Dogan A, Isaacson PG, Du MQ. t(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002;122:1286ŌĆō1294PMID : 11984515.


12. Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, Pan L, Crook T, Hamoudi R, Isaacson PG, Dyer MJ. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999;96:35ŌĆō45PMID : 9989495.


13. Zhang Q, Siebert R, Yan M, Hinzmann B, Cui X, Xue L, Rakestraw KM, Naeve CW, Beckmann G, Weisenburger DD, Sanger WG, Nowotny H, Vesely M, Callet-Bauchu E, Salles G, Dixit VM, Rosenthal A, Schlegelberger B, Morris SW. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat Genet 1999;22:63ŌĆō68PMID : 10319863.


14. Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, Yamaoka S, Seto M, Nunez G. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem 2001;276:19012ŌĆō19019PMID : 11262391.


15. Neumeister P, Hoefler G, Beham-Schmid C, Schmidt H, Apfelbeck U, Schaider H, Linkesch W, Sill H. Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas. Gastroenterology 1997;112:1871ŌĆō1875PMID : 9178679.


16. Peng H, Chen G, Du M, Singh N, Isaacson PG, Pan L. Replication error phenotype and p53 gene mutation in lymphomas of mucosa-associated lymphoid tissue. Am J Pathol 1996;148:643ŌĆō648PMID : 8579126.


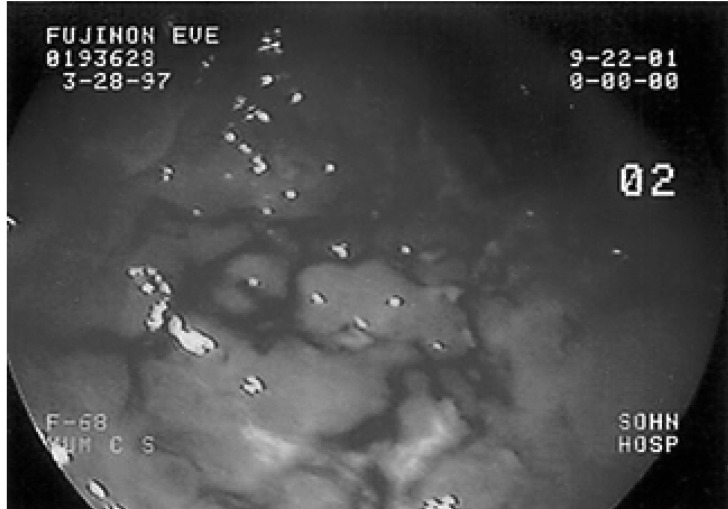
Figure┬Ā1
Gastroscopy showed ulcero-infiltrative lesions with a hemorrhagic and irregular surface located at the greater curvature side of the antrum.
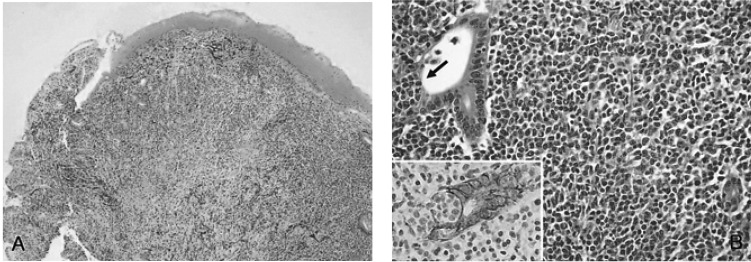
Figure┬Ā2
(A) Low power view showed mucosal ulceration with diffuse infiltration of lymphoid cells (H&E, ├Ś40). (B) High power view showed diffuse infiltration of monotonous centrocyte-like cells and a few large lymphoid cells with a conspicuous lymphoepithelial lesion (arrow), which are highlighted by the cytokeratin immunostain (Inset; Cytokeratin, ├Ś400) and demonstrate characteristics of MALT lymphoma (H&E, ├Ś400).
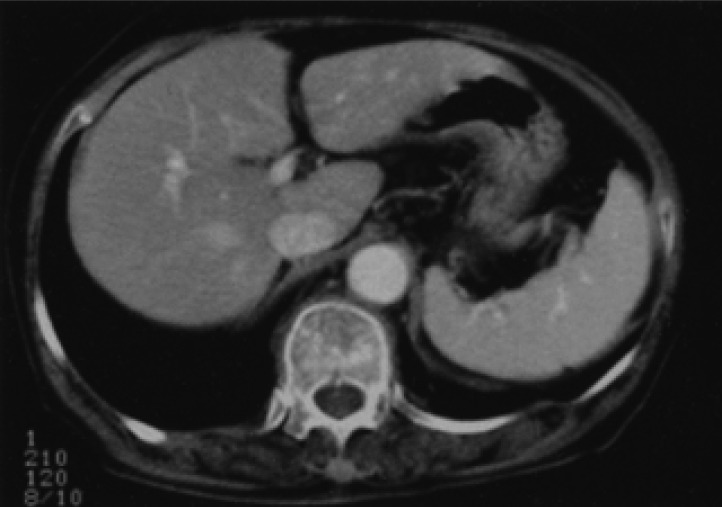
Figure┬Ā3
Abdominal CT scan performed four years after initial presentation showed neither gastric wall thickening nor lymph node enlargement. Moreover, there was no evidence of a hepatic mass.
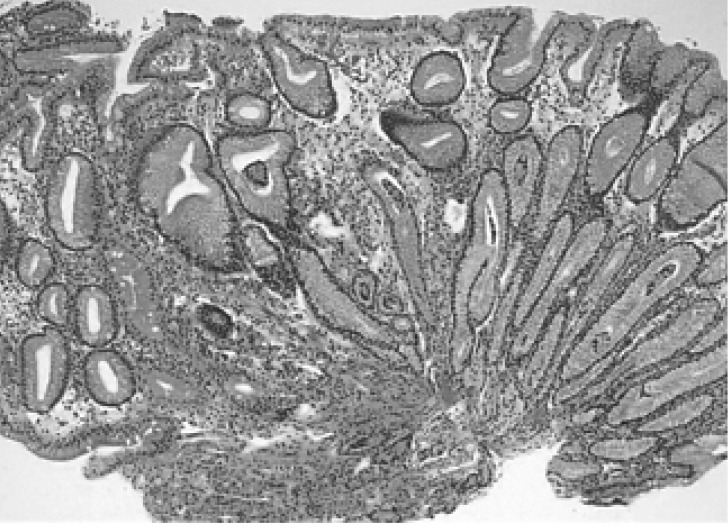
Figure┬Ā4
Gastric biopsy performed six years later, after initial presentation, showed chronic gastritis with intestinal metaplasia devoid of residual MALT lymphoma (H&E, ├Ś100)
Figure┬Ā5
T2 scan of a liver MRI showed multiple hepatic nodules in segments III, IV, VI, VIII (white arrow) with multiple lymphadenopathy (black arrow).
-
METRICS

- Related articles
-
Duodenal Mucosa-Associated Lymphoid Tissue Lymphoma: A Case Report2007 December;22(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


