INTRODUCTION
Pulmonary pneumatoceles are thin-walled, gas-filled cysts that develop within the lung parenchyma. They can be single emphysematous lesion, but are more often multiple. Most often, they occur as a complication of acute pneumonia, commonly caused by Staphylococcus aureus1, 2). However, pneumatoceles are also caused by other agents, including Streptoccus pneumoniae3), Haemophilus influenza5), Pseudomonas aeruginosa5), Escherichia coli6), Klebsiella pneumonia5, 6), Mycobacterium tuberculosis7), Proteus mirabilis8), Serratia marcescens6) and Pneumocystis jiroveci,9) and also by trauma10) and hydrocarbon-induced pneumonia11).
CASE REPORT
A 64-year-old man was admitted with a history of severe right pleuritic chest pain of a few days duration. He had been treated for diabetes mellitus and hypertension for twenty years, which were well controlled, without any complications. He also had a history of pulmonary tuberculosis, but denied alcoholism and drug abuse.
On admission, he had respiratory symptoms, including a mild cough, yellowish sputum and severe right pleuritic chest pain. He also had mild fever, with a body temperature of 37.3Ōäā, pulse rate 103/min, respiratory rate 18/min and blood pressure 150/80 mmHg. A chest examination revealed decreased breathing sound in the right lower lung. Laboratory studies revealed the following values: leukocytes 21,500/cu mm with 84% segmental neutrophil, 3% band forms, 10% lymphocyte and 4% monocytes; arterial pH 7.41, PCO2 37.5 mmHg, PO2 60.7 mmHg on room air.
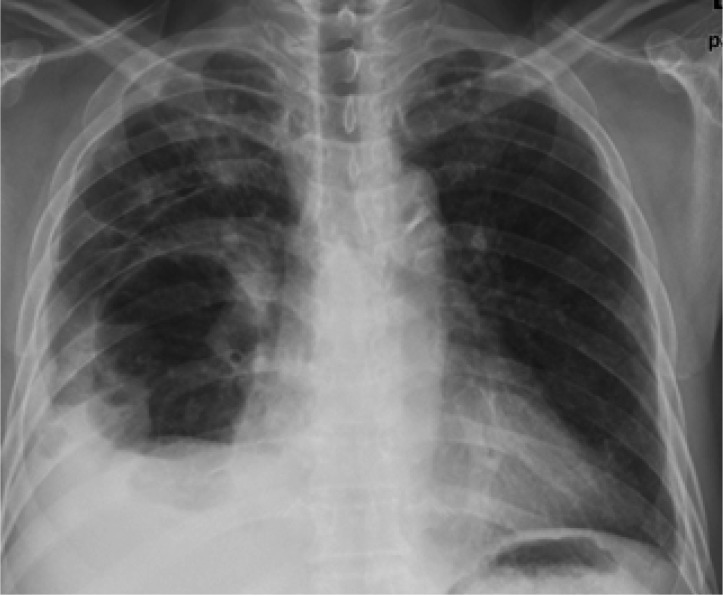
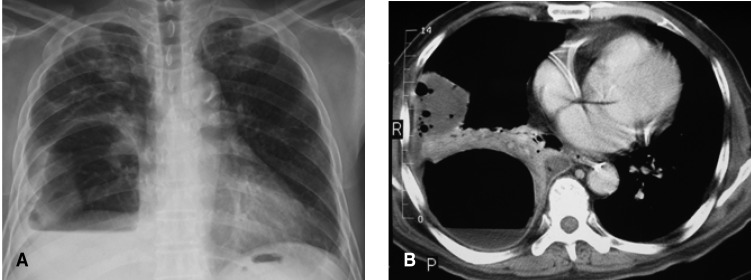
The chest radiography taken on admission revealed a loculated pleural effusion, with suspicious consolidation in the right lower lung. A blood culture was obtained and treatment was started with intravenous antibiotics (cefpiramide 1 g every 12 hours and amikacin 500 mg every 24 hours). Sono-guided thoracentesis was performed. The pleural fluid contained a pus-like material: WBC > 1,000/mm3 almost PMNL; glucose 346 mg/dL; protein 4.3 mg/dL; LDH 2211 u/L. Bacteria, fungi and tuberculosis cultures were all negative, as were the blood and sputum cultures. On the fifth day of hospitalization, chest radiography revealed an air cyst and air fluid level in the right lower lung (Figure 1). On the ninth day, the size of the air-cyst had rapidly increased to about 9├Ś11 cm in size; the air fluid level had also increased (Figure 2A).
Computed tomography of the chest was performed, and the enhanced chest CT scan showed a 9├Ś11 cm-sized thin-walled cavitary lesion, with an air fluid level, pleural effusion and consolidation in the right lower lung (Figure 2B).
A percutaneous pig-tail catheter was inserted into the pneumatocele with the air fluid level, and foul-odored pus drained. The bacterial culture was positive for Bacteroid species, Peptostreptococcus asaccharolyticus and Fusobacterium species. On the same day, antibiotics were changed to intravenous clindamycin, 600 mg every 8 hours. On the next day, after percutaneous catheter drainage, the patient became afebrile, and the size and amount of the air fluid level of the pneumatocele progressively decreased. On the twenty-seventh day, there was no further drainage and the catheter was removed. Eventually, the patient was discharged on the thirty-sixth day of hospitalization.
DISCUSSION
A pulmonary pneumatocele can occur in association with acute pneumonia, and is almost transient as the lesions generally resolve spontaneously and completely without sequelae1, 2, 5). Pneumatoceles are differentiated from lung abscesses due to their tendency to rapidly change in appearance, size and location1).
The precise pathogenesis and pathological anatomy of pneumatoceles are in most instances uncertain13). Recently, Quigley and Fraser proposed a pathogenesis, which after the cavity had formed, irritation and inflammation of a small draining bronchiole led to the formation of a mucus flap, focally adherent to the airway wall. The intracavitary portion, being mobile, could alternatively open and close the bronchiolar orifice, effectively acting as a check-valve13).
Radiological evidence of pneumatoceles most often occurs on the fifth to sixth day of hospitalization5), but on rare occasions may be visible on the initial chest radiograph. The usual medical care of pneumatoceles in the course of pneumonia is the treatment of the underlying pneumonia, but tension pneumatoceles, pneumothorax and infected pneumatoceles, which can be life-threatening, may require surgical intervention, such as catheter drainage and an operation12).
In our patient, the pneumatocele formed on the fifth day of hospitalization, and the size and amount of the air fluid level gradually increased. Large pneumatoceles can cause displacement and compression of the adjacent lung, resulting in cardiopulmonary insufficiency, but our patient had no pneumatocele-related symptoms. In most circumstances, pneumatoceles are asymptomatic and do not require surgical intervention. The majority of pneumatoceles resolve spontaneously over weeks to months, and without significant clinical or radiographic sequelae1, 2).
Treatment of the underlying pneumonia with antibiotics is the first line of therapy. Percutaneous catheter drainage is helpful, both as a diagnostic and therapeutic procedure for the pneumatocele and its complications12, 14). In our case, percutaneous catheter drainage of the pneumatocele infected by the mixed anaerobes was performed. It is also helpful to diagnose and treat the pneumatocele. Despite our efforts, we could find no similar case of anaerobic pneumonia complicated by pneumatoceles.
In summary, we report a case of an adult patient with anaerobic pneumonia, complicated by a pneumatocele, with secondary infection. The infected pneumatocele was successfully decompressed and drained using percutaneous catheter drainage. Our patient was successfully treated with antibiotics and percutaneous catheter drainage.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





