 |
 |
| Korean J Intern Med > Volume 20(4); 2005 > Article |
|
Abstract
Background
Changes in airway mucosal osmolarity are an underlying mechanism of bronchoconstrictive responses to exercise and hypertonic saline (HS). The purpose of this study was to examine whether an osmotic challenge test using HS can predict exercise-induced bronchospasm (EIB) in asthma patients.
Methods
Thirty-six young male asthmatic patients underwent bronchial challenge tests based on 4.5% HS, exercise (> 24h later), and methacholine (MCh) at the Chonnam National University Hospital. The relationships between responses to HS and exercise, and between MCh and exercise were evaluated.
Results
The maximal fall in forced expiratory volume in one second following exercise was significantly higher in the HS-responders (n=19) than in the HS-nonresponders (n=17, 35.9┬▒4.1% vs. 17.9┬▒2.7%, p<0.001), and there was a significant correlation between the severity of EIB and HS-airway hyperresponsiveness (AHR). When compared with the MCh-AHR test in terms of predicting EIB, the HS-AHR test showed higher specificity (71.4% vs. 42.9%), but a lower sensitivity (58.6% vs. 89.7%) and negative predictive value (29.4% vs. 50.0%). At the moderate AHR cutoff value, the MCh-AHR test had a specificity that was comparable with and predictive values that were higher than those of the HS-AHR test.
Conclusions
The HS-AHR test was more specific than the MCh-AHR test, but was less sensitive and had a poorer negative predictive value, which in combination preclude the use of the HS-AHR test as a screening tool for EIB. The MCh-AHR test had a cutoff value for moderate AHR that may be more useful for predicting EIB in asthmatic patients.
Although the precise mechanisms underlying exercise-induced bronchoconstriction (EIB) in asthma are unknown, it has been suggested that a change in the osmolarity of airway lining fluids and the consequent release of various chemical mediators, which occur during the warming and humidification of inhaled air and evaporation of airway mucosal surface water, are important provoking factors1). In addition, reactive hyperemia of the bronchial microvasculature, which results in changes in airway diameter and is promoted by the thermal effects of exercise (i.e., an initial airway cooling and subsequent rapid rewarming), is considered to be another cause of bronchoconstriction.
Airway hyperresponsiveness (AHR) to direct stimuli, such as methacholine (MCh) or histamine, also occurs non-asthmatic diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, and irritable bowel disease2). However, in asthma, indirect stimuli (exercise, hyper- or hypotonic aerosols, isocapnic hyperventilation, sodium metabisulphite/SO2, propranolol, adenosine, bradykinin, and tachykinins) stimulate inflammatory or neuronal cells to release chemical mediators, and thus AHR to indirect stimuli is considered to be more specific for asthma3). AHR to adenosine is more specific for diagnosing asthma4) and is better at predicting sputum eosinophilia5), corticosteroid sensitivity6), and responsiveness to allergen-avoidance at high altitude7) in asthmatic patients. We previously reported that AHR to hypertonic saline (HS), as opposed to MCh, is more closely related to eosinophil numbers, IL-5 level, and the interferon-╬│/IL-5 ratio in sputum8).
Both exercise and HS are indirect provocative stimuli that can cause changes in the osmolarity of airway-lining fluids. Therefore, it is speculated that an HS provocation test could predict EIB in asthmatic patients. However, no comparative study of the various bronchial provocation tests has been performed in Korea to date, except a study by Cho et al.9), which showed increased MCh-AHR after an exercise provocation test in asthmatic patients.
The purpose of this study was to examine whether the osmotic challenge test using HS can predict EIB in asthmatic patients.
The study group consisted of 36 young male asthma patients (18-23 years old), who consecutively visited the Allergy Clinic at Chonnam National University Hospital, Gwangju, Korea. Asthma was defined by the presence of asthmatic symptoms (cough, dyspnea, and wheezing) and by a 20% fall in forced expiratory volume in one second (FEV1) in response to an MCh concentration of <16 mg/mL.
Routine laboratory tests including peripheral blood eosinophil counts, serum IgE level determinations, and allergy skin prick tests were performed. The patients underwent bronchial challenge tests using HS, and after at least 24 h had elapsed exercise followed by MCh tests were performed. By investigating the relationships between exercise and HS or MCh, test results were evaluated to identify the test more specific for the diagnosis of EIB.
Lung function was measured with a spirometer (Spiro Analyzer ST-250, Fukuda Sangyo, Tokyo). The tests were conducted using techniques that met the standards developed by the Intermountain Thoracic Society10). The largest sum of FEV1 and forced vital capacity from more than three acceptable flow-volume curves was selected as being representative. Medications, such as, anti-histamine (>48 h), theophylline (>24 h), long-acting ╬▓2 agonists (>24 h), ipratropium (>12 h), and short-acting ╬▓2 agonists (>8 h) were stopped before the bronchial provocation tests. Airway responsiveness to HS solution was assessed using the method described by Iredale et al.11) using 4.5% NaCl aerosols delivered from an ultrasonic nebulizer (Ultra-neb 2000, Devilbiss, PA) through a Hans Rudolph 2700, two-way, non-rebreathing valve box to a rubber mouthpiece whilst subject wore nose clips. Subjects inhaled 4.5% NaCl for successively doubled periods (i.e., 0.5, 1.0, 2.0, 4.0, and 8.0 min). FEV1 was measured at 30 and 90s after each inhalation period using a spirometer. The next challenge period followed within 3 min of the end of the previous period. If FEV1 fell by less than 10%, the exposure time was doubled; if FEV1 fell between 10 and 15%, the exposure time was kept the same, but if the FEV1 fall exceeded 15%, or if the a subject reached the maximal dose without showing a fall exceeding 15%, the challenge was stopped. Dose-response curves were constructed by plotting the percentage change in FEV1 against the cumulative aerosol dose delivered (expressed in mL) on a log scale for each inhalation period. Provocative doses of HS causing a 15% fall in FEV1 (PD15) were calculated by linearly interpolating log-dose-response curves. The degree of airway responsiveness to HS was categorized as severe AHR (Grade 3) for PD15 Ōēż 2 mL, moderate AHR (Grade 2) for PD15 > 2 but Ōēż 6 mL, mild AHR (Grade 1) for PD15 > 6 but Ōēż 20 mL, and normal (Grade 0) for PD15 >20 mL12). Patients who did not respond to the highest dose of HS were assigned a value that was twice that of the highest dose applied.
The MCh challenge test was conducted as for the standardized tidal breathing method of Corkcroft et al.13). MCh in isotonic saline was aerosolized at room temperature in a Devilbiss 646 nebulizer (Devilbiss Co., Somerset, PA; output 0.13 mL/min). The dilution increments used were 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, 10, and 25 mg/mL. The aerosols were inhaled by tidal breathing through the mouth with the nose clipped over 2 min at 5-min intervals. Challenge testing was discontinued if the FEV1 dropped by 20% or more from baseline or if the maximal concentration of MCh had been administered. The provocation concentration of MCh resulting in a 20% fall in FEV1 (PC20) was calculated by linearly interpolating log-dose-response curve.
Free running tests to detect EIB in asthma were performed for 6 min, as previously described14). Each subject checked his radial artery pulse rate while running. Patient accelerated to as to raise the pulse rate to 85% of the predicted maximum (calculated as 220 minus age in years) during the first 2-3 min of exercise. For the remaining exercise time, subjects ran at a constant running speed. Immediately after running, subjects' pulse rates (> 85% of the predicted maximum) were confirmed using a pulse oximeter (Symphony N-3000, Nellcor Puritan Bennett, CA). FEV1 values were measured immediately before and at 1, 3, 5, 7, 10, 15, 20, and 30 min after exercise. Maximal EEV1 change was calculated as 100% ├Ś [(pre-exercise FEV1 - lowest post-exercise FEV1)/pre-exercise FEV1]. A positive EIB was defined as Ōēź a 15% reduction in post-exercise FEV1 versus pre-exercise FEV1.
Twenty-nine (80.6%) of 36 asthmatic patients showed a Ōēź 15% reduction in FEV1 after exercise. Patients showing a positive response to exercise had higher peripheral blood eosinophil counts (6.5┬▒0.7% vs. 3.4┬▒0.4%, p<0.01) and lower MCh-PC20 values (geometric mean: 0.37 mg/mL vs. 2.10 mg/mL, p<0.05) than patients with a negative response (Table 1).
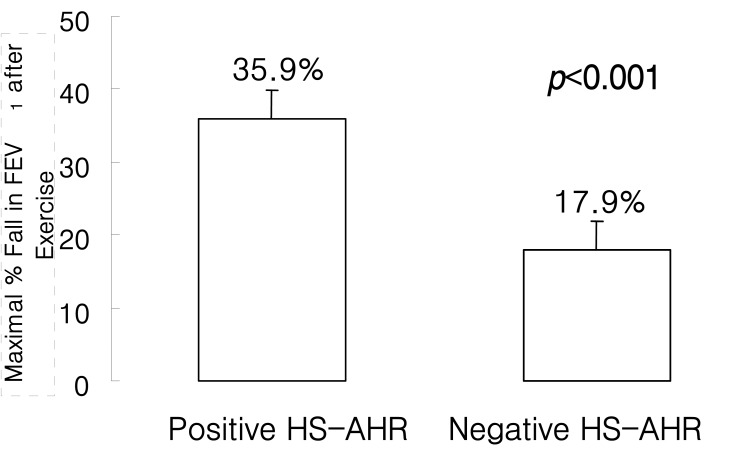
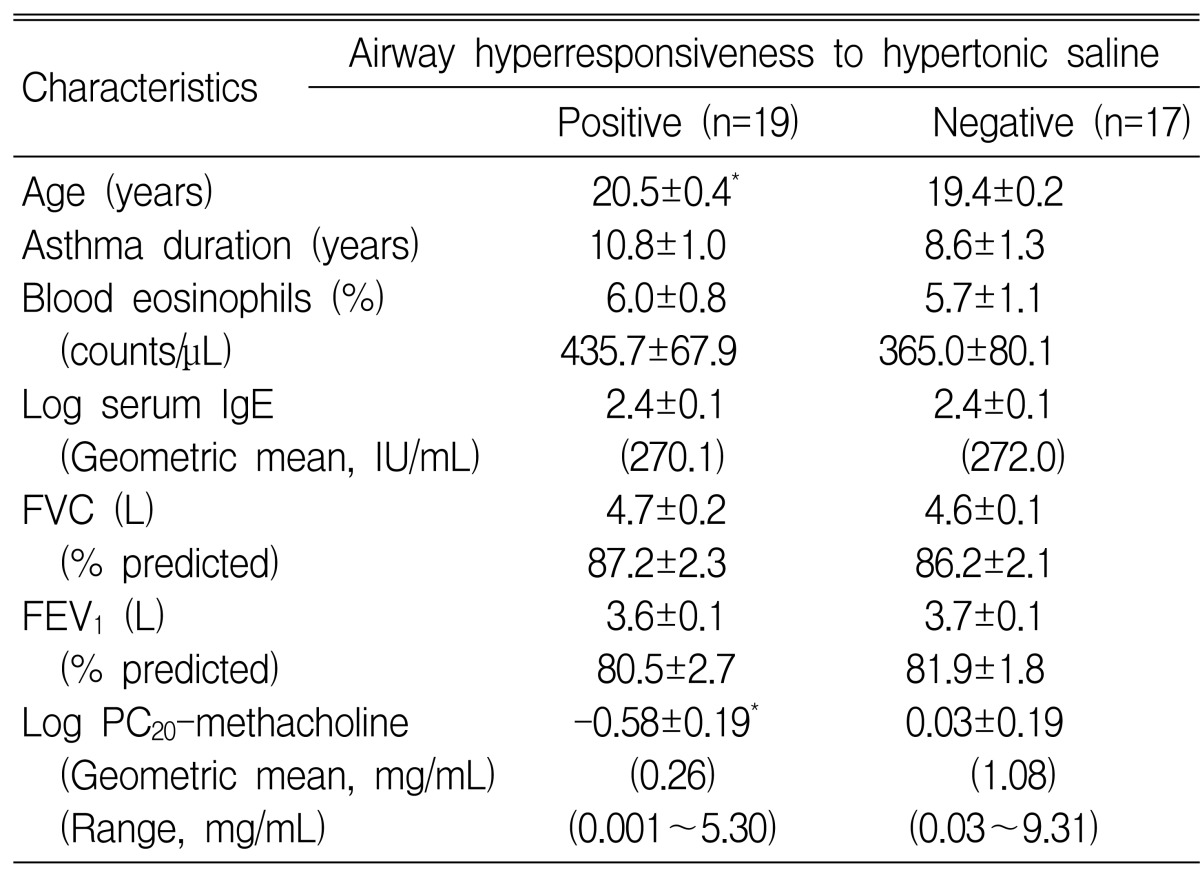
When the 36 patients were classified according to a positive or negative AHR response to the 4.5% HS inhalation test, 19 (52.8%) proved positive, i.e., the provocative dose of HS causing a 15% fall in FEV1 was Ōēż 20 mL. Table 2 compares the characteristics of subjects positive or negative for HS-AHR. Patients with a positive HS-AHR were significantly older and had more severe MCh-AHR (MCh-PC20: 0.26 mg/mL vs. 1.08 mg/mL, p<0.05) than those with negative HS-AHR. However, when subject age was restricted to 22 years old, no significant relation was observed between HS-AHR results and age, but there was a significant difference in MCh-AHR (0.17 mg/mL vs. 1.08 mg/mL, p<0.01) between the positive (n=15) and negative (n=17) HS-AHR groups. The maximal percentage fall in FEV1 after exercise was significantly higher in patients with positive HS-AHR than in those with negative HS-AHR (35.9┬▒4.1% vs. 17.9┬▒2.7%, p<0.001) (Figure 1).
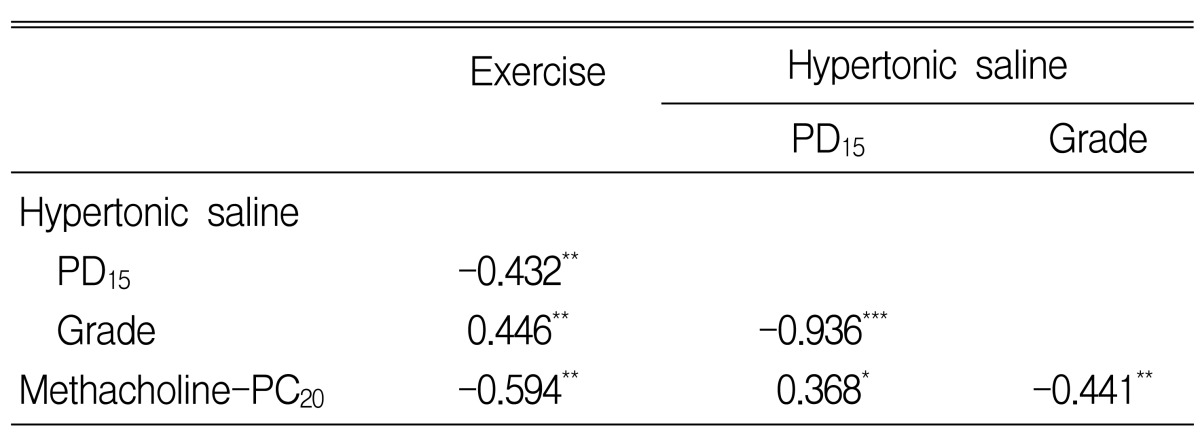
The maximal percentage fall in FEV1 after exercise was significantly related to HS-AHR (Log PD15) (Figure 2) and MCh-AHR (Log PC20) (Table 3).
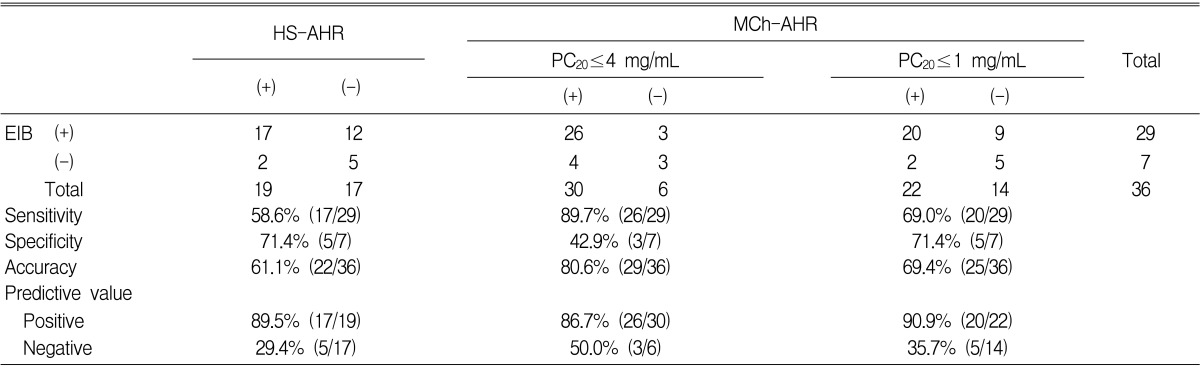
The specificity for the presence of EIB was significantly higher for the HS-AHR test than for the MCh-AHR test (cut-off value of MCh-PC20 Ōēż 4 mg/mL15)) (71.4% vs. 42.9%), but was comparable to that in the test at a cut-off value of Ōēż 1 mg/mL for moderate to severe MCh-AHR15) (71.4% vs. 71.4%) (Table 4). However, both the sensitivity (58.6% vs. 89.7%) and accuracy (61.1% vs. 80.6%) of the HS-AHR test for EIB were lower than those of the MCh-AHR test. Moreover, the negative predictive value of the HS-AHR test was poorer than that of the MCh-AHR test (29.4% vs. 50.0%). When the cut-off value of the MCh-AHR test was set at 1 mg/mL, both the positive and negative predictive values of the MCh-AHR test for EIB were higher than those of the HS-AHR test, respectively (positive: 90.9% vs. 89.5%, negative: 35.7% vs. 29.4%).
In this study, AHR to HS was found to be related to the degree of bronchoconstriction after exercise, and the HS-AHR test showed high specificity for the diagnosis of EIB. AHR to MCh was also found to be related to the degree of bronchoconstriction after exercise, but the specificity of the MCh-AHR test was poor and was comparable to that of the HS-AHR test only at a cut-off value of Ōēż 1 mg/mL for moderate to severe MCh-AHR.
As it has been reported that 70~80% of patients with asthma show a positive response to the exercise test1), and because asthma is a common disease, general physicians would probably treat EIB and asthma patients in clinics. A standardized exercise test has been developed, but there are difficulties associated with test application in general practice, and thus, several other tests have been examined as potential substitutes. Although the MCh challenge test is highly sensitive for asthma detection, it is well known that the MCh challenge test produces false positives for many types of non-asthmatic diseases, and it has been suggested that several challenge tests using indirect stimuli may be more specific for asthma. The HS challenge test works by causing a change in the osmolarity of airway-lining fluids in a manner similar to exercise. In addition, the ease of test administration and of obtaining a dose-response curve, and the ability of HS to induce sputum with further inhalation make the HS challenge test an attractive means of simultaneously evaluating both AHR and airway inflammation, which are the two characteristic features of asthma16). We previously reported that AHR to HS (an indirect stimulus), as compared with AHR to MCh (a direct stimulus), might better reflect asthmatic airway inflammation. As an extension of this concept, we investigated whether HS-AHR or MCh-AHR is more closely related to EIB.
Belcher et al.17) showed that EIB is significantly related to airway responsiveness to HS but not to histamine. Makker and Holgate18) showed that in 29 asthmatic patients with a history of EIB, 23 (79%) were responsive to 3.6% NaCl. They also found that neither histamine nor MCh-PC20 were significant correlation with EIB symptom severity scores or responsiveness to HS. Moreover, Belcher et al.19) showed that exercise and 3.6% aerosolized HS induced refractoriness interchangeably under repeated examination, whereas histamine-induced bronchoconstriction did not induce a refractory period to EIB.
Both EIB and HS-AHR seem to occur in patients with severe asthma. Atopy, defined as a positive skin test to allergens, especially Dermatophagoides pteronyssinus, contributes to the development of exercise-induced bronchospasm independently of AHR to MCh20). Peripheral blood eosinophil count21), eosinophil cationic protein concentration22, 23), leukotriene C4 production by eosinophils24), airway eosinophil count, and eosinophil cationic protein concentration25, 26) have been shown to be correlated with EIB. In the present study, peripheral blood eosinophil counts were also found to be related to EIB. Chae et al.25) showed that EIB is more related to sputum eosinophil counts than with atopy. In addition, in the present study, patients with EIB had significantly more severe MCh-AHR. Patients with positive HS-AHR also had higher peripheral blood eosinophil counts and significantly more severe MCh-AHR than those with a negative HS-AHR. Smith and Anderson27) showed that asthmatic patients recording a fall in FEV1 of Ōēź20% to 4.5% saline had a PD20 to MCh of < 2 ┬ĄM, which is a response consistent with moderate to severe AHR. Because an MCh-PD20 of <2 ┬ĄM is comparable to an MCh-PC20 of <4 mg/mL, their results concur with our result of an MCh-PC20 of 5.30 mg/mL for patients with HS-AHR. However, the geometric mean MCh-PC20 was 1.08 mg/mL even in patients without HS-AHR; thus, HS-AHR seems to occur when MCh-AHR is moderate to severe (i.e., MCh-PC20 <1 mg/mL), according to the American Thoracic Society's grading system15).
AHR to distilled water, another osmotic stimulus, was also found to be significantly correlated with EIB, and cromolyn was found to block both distilled water-induced bronchospasm and EIB in patients with asthma, which suggests the presence of a similar mechanism28). However, the sensitivity of the AHR test using HS is higher than that based on distilled water29), and HS inhalation can be used to simultaneously test for AHR and airway inflammation, the characteristic features of asthma16). Therefore, an investigation into the relationship between HS-AHR and EIB would be worthwhile. In Korea, only one study by Cho et al.30) has been conducted using distilled water, in which it was observed that distilled water inhalation caused coughing without a deterioration in lung function in cough variant asthma.
Although the specificity of the HS-AHR test was high for the diagnosis of EIB, its low sensitivity and poor negative predictive value suggests that the test would be inappropriate as a screening tool. MCh-AHR, but not HS-AHR, was significantly different for asthmatic patients with EIB and without EIB. Moreover, both positive and negative predictive values were higher for the MCh-AHR test than for the HS-AHR test at a MCh-PC20 cutoff value of <1 mg/mL. Therefore, the MCh-AHR test was found more useful than the HS-AHR test at predicting EIB. The diagnostic accuracy or total value of a test for diagnosis can be evaluated in terms of specificity plus sensitivity31), and in the present study, the accuracy of the
MCh-AHR test was greater than that of the HS-AHR test.
We speculate that EIB may be more closely related to HS-AHR than with MCh-AHR because the underlying mechanisms of HS-AHR and EIB appear similar, and both appeared in asthmatic patients with moderate to severe MCh-AHR. However, even though the specificity of the HS-AHR test was comparable to the MCh-AHR test for moderate AHR its sensitivity and negative predictive values were poor, which preclude its use as a screening tool for EIB. Thus, at a cutoff value corresponding to moderate AHR, MCh-AHR may be more useful that HS-AHR for predicting EIB in asthmatic patients.
Notes
This paper was supported by the Chonnam University Hospital Research Institute of Clinical Medicine (Grant No. CUHRI-U-200354).
References
1. Godfrey S. In: Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ, eds. Exdercise-induced asthma. Asthma. 1997;Philadelphia: Lippincott-Raven Publishers, 1105ŌĆō1119.
2. O'Byrne PM. In: Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, eds. Airway hyperreponsiveness. Allergy principles & practice. 1998;5th ed. Toronto: Mosby, 859ŌĆō866.
3. van Schoor J, Joos GF, Pauwels RA. Indirect bronchial hyperresponsiveness in asthma: mechanisms, pharmacology and implications for clinical research. Eur Respir J 2000;16:514ŌĆō533PMID : 11028670.


4. Avital A, Springer C, Bar-Yishay E, Godfrey S. Adenosine, methacholine, and exercise challenges in children with asthma or paediatric chronic obstructive pulmonary disease. Thorax 1995;50:511ŌĆō516PMID : 7597663.



5. van den Berge M, Meijer RJ, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, Postma DS. PC20 adenosine 5'-monophosphate is more closely associated with airway inflammation than PC20 methacholine. Am J Respir Crit Care Med 2001;163:1546ŌĆō1550PMID : 11401871.


6. van den Berge M, Kerstjens HA, Meijer RJ, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Respir Crit Care Med 2001;164:1127ŌĆō1132PMID : 11673197.


7. Benckhuijsen J, van den Bos JW, van Velzen E, de Bruijn R, Aalbers R. Differences in the effect of allergen avoidance on bronchial hyperresponsiveness as measured by methacholine, adenosine 5'-monophosphate, and exercise in asthmatic children. Pediatr Pulmonol 1996;22:147ŌĆō153PMID : 8893252.


8. Choi IS, Hong SN, Lee YK, Koh YI, Jang AS, Lee HC. Asthmatic airway inflammation is more closely related to airway hyperresponsiveness to hypertonic saline than to methacholine. Korean J Intern Med 2003;18:83ŌĆō88PMID : 12872444.



9. Cho YJ. The nonspecific bronchial hyper-reactivity during refractory period following exercise in exercise-induced asthma. Ewha Med J 1998;21:73ŌĆō79.

10. Morris AH, Kanner RE, Crapo RO, Gardner RM. Clinical pulmonary function testing: a manual of uniform laboratory procedures. 1984;2nd ed. Salt Lake City: Intermountain Thoracic Society.
11. Iredale MJ, Wanklyn SA, Phillips IP, Krausz T, Ind PW. Non-invasive assessment of bronchial inflammation in asthma: no correlation between eosinophilia of induced sputum and bronchial responsiveness to inhaled hypertonic saline. Clin Exp Allergy 1994;24:940ŌĆō945PMID : 7842363.


12. Anderson SD, Gibson P. Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ. Use of aerosols of hypertonic saline and distilled water (fog). Asthma. 1997;Philadelphia: Lippincott-Raven Publishers, 1135ŌĆō1149.
13. Cockcroft DW. Measurement of airway responsiveness to inhaled histamine or methacholine: method of continuous aerosol generation and tidal breathing inhalation. Proceedings from a workshop. 1985;Astra Pharmaceuticals Canada Ltd, 22ŌĆō29.
14. Koh YI, Choi IS. Seasonal difference in the occurrence of exercise-induced bronchospasm in asthmatics: dependence on humidity. Respiration 2002;69:38ŌĆō45PMID : 11844961.


15. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JR, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med 2000;161:309ŌĆō329PMID : 10619836.


16. Anderson SD. Exercise-induced asthma and the use of hypertonic saline aerosol as a bronchial challenge. Respirology 1996;1:175ŌĆō181PMID : 9424393.


17. Belcher NG, Lee TH, Rees PJ. Airway responses to hypertonic saline, exercise and histamine challenges in bronchial asthma. Eur Respir J 1989;2:44ŌĆō48PMID : 2707402.


18. Makker HK, Holgate ST. Relation of the hypertonic saline responsiveness of the airways to exercise induced asthma symptom severity and to histamine or methacholine reactivity. Thorax 1993;48:142ŌĆō147PMID : 8493628.



19. Belcher NG, Rees PJ, Clark TJ, Lee TH. A comparison of the refractory periods induced by hypertonic airway challenge and exercise in bronchial asthma. Am Rev Respir Dis 1987;135:822ŌĆō825PMID : 3565931.


20. Koh YI, Choi IS, Lim H. Atopy may be related to exercise-induced bronchospasm in asthma. Clin Exp Allergy 2002;32:532ŌĆō536PMID : 11972598.


21. Koh YI, Choi S. Blood eosinophil counts for the prediction of the severity of exercise-induced bronchospasm in asthma. Respir Med 2002;96:120ŌĆō125PMID : 11860169.


22. Cho SH, Jee YK, Yoon HJ, Min KU, Kim YY, Bahn JW, Lee BJ, Son JW, Lee SR, Kim YK, Lee MH. Role of eosinophils on the development of exercise-induced asthma. Korean J Allergy 1997;17:299ŌĆō306.
23. Bae WJ, Park JK, Lee JO, Kang IJ. A study of relationship between variations in serum eosinophil cationic protein (ECP) levels and bronchial hyperreactivity (BHR) in exercise-induced asthma. Pediatr Allergy Respir Dis 1996;6:71ŌĆō83.
24. Koh YY, Lee SY, Jeong JH, Kim CK, Park CH, Choi JH. Comparative study on the activation status of eosinophils in exercise- and allergen-induced asthma. Korean J Allergy 1997;17:286ŌĆō298.
25. Chae HS, Kim YJ, Song DY, Kang IJ. The effect of atopy and airway eosinophilic inflammation on exercise-induced bronchospasm in asthmatics. Pediatr Allergy Respir Dis 2003;13:81ŌĆō89.
26. Yoshikawa T, Shoji S, Fujii T, Kanazawa H, Kudoh S, Hirata K, Yoshikawa J. Severity of exercise-induced bronchoconstriction is related to airway eosinophilic inflammation in patients with asthma. Eur Respir J 1998;12:879ŌĆō884PMID : 9817162.


27. Smith CM, Anderson SD.. a comparison with responses to hyperpnoea, methacholine and water. Eur Respir J 1990;3:144ŌĆō151PMID : 2178963.


28. Bascom R, Bleecker ER. Bronchoconstriction induced by distilled water: sensitivity in asthmatics and relationship to exercise-induced bronchospasm. Am Rev Respir Dis 1986;134:248ŌĆō253PMID : 3090916.

29. Riedler J, Reade T, Dalton M, Holst D, Robertson C. Hypertonic saline challenge in an epidemiologic survey of asthma in children. Am J Respir Crit Care Med 1994;150:1632ŌĆō1639PMID : 7952626.


30. Cho YS, Lee JC, Lim YJ, Lee EY, Shin JH, Lim MK, Yoo B, Moon HB. Clinical features and cough sensitivity of patients with idiopathic chronic cough. J Asthma Allergy Clin Immunol 1999;19:188ŌĆō199.
Figure┬Ā1
Maximal post-exercise bronchospasm in asthmatics with (n=19) and without (n=17) airway hyperresponsiveness (AHR) to hypertonic saline (HS).
Figure┬Ā2
Relationship between airway sensitivity to hypertonic (4.5%) saline and maximal % fall in forced expiratory volume in one second after exercise in asthmatics.

Table┬Ā1
Comparisons of subject characteristics with respect to the presence and absence of exercise-induced asthma

Table┬Ā2
Comparisons of subject characteristics with respect to positive and negative airway hyperresponsiveness to hypertonic (4.5%) saline



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



