 |
 |
| Korean J Intern Med > Volume 20(3); 2005 > Article |
|
Abstract
Background
Although high dose chemotherapy coupled with an autologous stem cell transplantation (ASCT) is widely accepted as effective therapy for multiple myeloma (MM), few reports are available in Korea, especially in the area of double ASCT. We present the results of an institutional retrospective study of 12 patients with MM treated by double ASCT.
Methods
Eligible patients received induction therapy using vincristine, adriamycin, dexamethasone (VAD), and mobilization was performed using cyclophosphamide plus lenograstim. High-dose melphalan (total 200 mg/m2) was used to condition the ASCT.
Results
The median interval from diagnosis to ASCT was 6 months (range, 1.8-15.3 months). The median interval between the 1st and 2nd ASCT was 4.4 months (range 2.1-48.7 months). The median follow up was 18.3 months (range 8.1-50.5 months) for the nine surviving patients. No therapy-related mortality occurred. Following induction chemotherapy, two patients experienced CR. Following double ASCT, eight patients experienced CR. The 5 year OS was 59%. The median duration of event free survival was 2.13 years (95% CI, 0.84-3.42).
Multiple myeloma (MM) accounts for 14% of hematologic malignancies and frequently appears in the elderly1). The incidence of MM has increased in Korea since the 1980s1, 2). This increase may be partially attributed to the growing population of aged people and partially to improved detection rates.
The standard therapy using melphalan plus prednisolone (MP) appears to be ineffective, with few complete responses (CR; Ōēż5%) and a median survival of only 30 to 36 months3). There is no evidence that additional cytotoxic agents, such as adriamycin and nitrosourea, offer better survival rates compared with those achieved with MP4).
The failure of standard therapy to improve the outlook of MM has led to the treatment of this disease with high dose chemotherapy.
High doses of melphalan can induce a high rate of response, even in patients with refractory MM. However, this approach induced severe myelosuppression and a toxic mortality rate of 10%5). To shorten the myelosuppressive period and reduce therapy related mortality, autologous stem cell transplantation (ASCT) was used following high dose therapy (HDT), and have been shown to reduce myelotoxicity6).
Recently, a prospective randomized trial conducted by the French Myeloma Group demonstrated that HDT coupled with ASCT is superior to the standard therapy in terms of response rate (RR), event-free survival (EFS), and overall survival (OS)7).
In addition, dose intensification with double ASCT markedly augments tumor reduction, resulting not only in higher CR rates, but also significantly extending EFS and OS, compared to historical single ASCT8).
More recently, a randomized trial of either one or two successive ASCT for MM was reported, and showed that double ASCT improved overall survival of patients with MM9).
Although HDT with ASCT is widely accepted as an effective and safe consolidation therapy for MM, few reports are available in Korea10), especially in the area of double ASCT. We present the results of an institutional retrospective study involving 12 patients with MM treated with HDT and double ASCT.
Between November 1996 and June 2003, all MM treated by HDT and double ASCT were reviewed. Eligibility criteria for HDT and double ASCT included symptomatic MM, an upper age limit of 70 years and adequate cardiopulmonary function. At protocol entry, exclusion criteria was poor performance status (ECOG >2) related to MM.
VAD induction chemotherapy was chosen due to its rapid tumor cytoreduction without dose alteration in patients who have renal dysfunction, and for its lack of stem cell toxicity (VAD: vincristine 0.4 mg by continuous intravenous (iv) infusion for 4 days (total 1.6 mg) and adriamycin 9 mg/m2 by continuous iv infusion for 4 days (total 36 mg/m2) and dexamethasone 40 mg once a day p.o on days 1-4 every 4 weeks for 3-5 cycles).
Mobilization was performed by chemotherapy plus lenograstim. The chemotherapy used for mobilization was cyclophosphamide 4 g/m2. On the next day of chemotherapy, lenograstim at 10 ug/kg/day was administered subcutaneously and continued until the completion of leukapheresis was observed. Peripheral blood hematopoietic cells (HPC) and harvested CD34+ cells were enumerated using an automated hematology analyzer (Sysmex SE-9000TM) and flow cytometry, respectively. Circulating HPC were monitored daily and when they reached a count of 5/mm3 or more, after nadir, autologous stem cell harvest began. Leukapheresis was performed by a large volume apheresis procedure using a Fenwal CS3000 plus cell separator via a large bore catheter. Peripheral blood stem cells were harvested with a target of 10.0├Ś106/kg CD34+ cells.
HDT was performed with melphalan (100 mg/m2 iv infusion for 30min for two consecutive days: day -3 and -2; total 200 mg/m2) at least 4 weeks after mobilization. Stem cells were infused on day 0. Subcutaneous lenograstim 5 ug/kg/day started on day 1 and continued until the absolute neutrophil count (ANC) reached 1,000/mm3 for two consecutive days. The 2nd ASCT was performed within 3-9 months following the 1st ASCT, with the exception of one case which had a 4 year interval. The 2nd ASCT followed the exact same process as that of 1st ASCT. Prophylactic antibiotics, antifungal and antiviral agents were used. Hematologic and non-hematologic toxicities were graded according to the modified WHO criteria.
All patients were evaluated at each cycle of chemotherapy and when the autologous stem cell collection and ASCT were performed. Assessments included a physical examination, complete blood count, biochemistry profile, serum and/or urine protein electrophoresis and its quantification, serum and urine immunoelectrophoresis, and immunofixation.
CR was defined as the disappearance of monoclonal immunoglobulin (Ig) in serum and/or urine on immunofixation analysis. Partial response (PR) was defined as both a minimum of 50% reduction of the initial serum M-protein concentration and either a reduction of Bence-Jones proteinuria to less than 0.2 g/24 h or a greater than 75% reduction in urinary Bence-Jones protein. Stable disease (SD) was defined as no change or a decrease of less than 50% in paraprotein levels. Progressive disease (PD) was defined as a confirmed increase in the serum paraprotein concentration by more than 25% from the level at the time of the best response, an increase of Bence-Jones proteinuria to more than 1.0 g/24 h, or other unequivocal signs of disease progression, such as hypercalcemia, progressive skeletal disease, or soft tissue plasmacytoma. Relapse was defined as the reappearance of monoclonal Ig in patients who achieved CR, and an increase of greater than 25% in monoclonal M-protein for non-CR patients, or other unequivocal signs of disease progression such as hypercalcemia or the development of new extramedullary disease.
Responses were evaluated 4 weeks after ASCT. Protein electrophoresis and immunofixation of serum and/or urine were performed. If CR was achieved, the patient was evaluated every month for the first two months, then every two months on three occasions, and every three months thereafter. If CR was not achieved initially, the patient was evaluated every month until CR was achieved. At each follow-up visit, the patients' history was recorded and they underwent a physical examination, complete blood count, platelet count and liver function test. Serum and/or urine protein electrophoresis were screened. If a relapse or progression was confirmed, the decision of whether to perform further therapy or not was at the discretion of the participating physicians.
An event was defined as progression, relapse, or death due to any cause without progression. EFS was calculated for all patients from the date of diagnosis to the time of disease progression, relapse, or death, or until the date the patient was last known to be in remission. OS was calculated from the date of diagnosis to death or the date the patient was last known to be alive. Survival rates were plotted by the Kaplan-Meier method. A p value <0.05 was considered significant. All calculations were performed on SPSS, version 10.1.
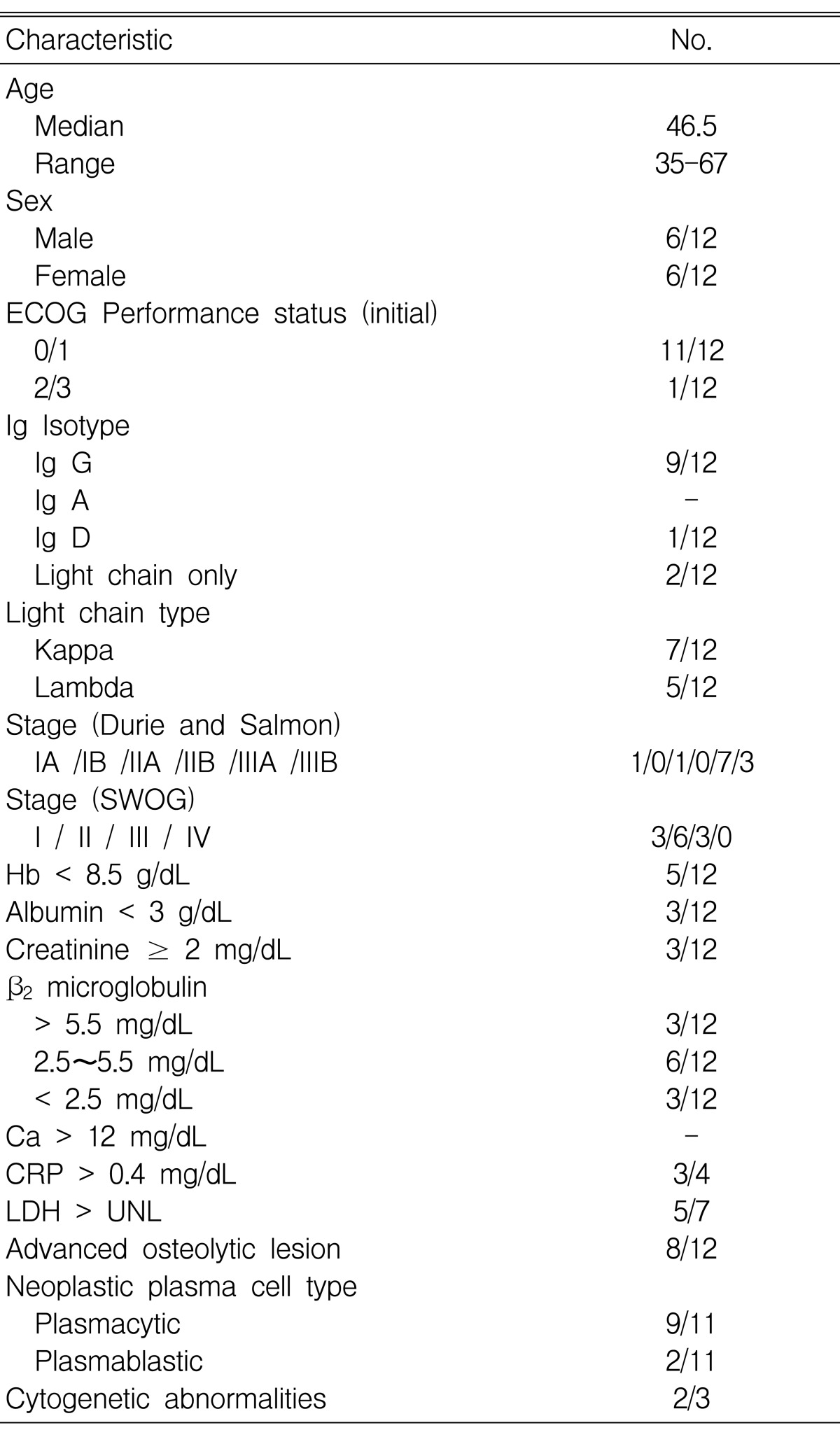
Table 1 shows the base-line characteristics of the 12 patients. Six were men and six were women. The study population was divided into two groups based on the patients' response after the 1st ASCT. At the 2nd ASCT, the two groups were each comprised of six patients; the 1st group (favorable group) . did not display progression and/or relapse after the 1st ASCT, the 2nd group (unfavorable group) - displayed progression or relapse. The median age of the patients was 46.5 years (range, 35-67). The most common isotype of the monoclonal Ig was Ig G in nine cases. At diagnosis, seven patients had stage IIIA disease, three had stage IIIB, and two had stage II or less according to classification by Durie and Salmon. According to the SWOG staging system11), three patient had stage I, six patients had stage II and three patients had stage III. Five patients had severe anemia (hemoglobin < 8.5 g/dL). Three patients had hypoalbuminemia (albumin < 3 g/dL). Three patients had azotemia (Cr Ōēź 2 mg/dL). The serum ╬▓2 microglobulin level was above 5.5 in three patients, between 2.5 and 5.5 in six patients, and below 2.5 mg/dL in three patients. Three patients were checked for cytogenetic abnormalities. Two patients showed abnormalities (Table 2).
Transplant specifics are shown in Table 3. There were no harvest failures during autologous stem cell collection. The median harvesting time was 4 days (range, 2-8 days). The median interval between diagnosis and the first ASCT was 6 months (range, 1.8-15.3 months). And the median interval between the 1st and the 2nd ASCT was 4.4 months (range, 2.1-48.7 months). In the 1st ASCT, median infused mononuclear cells and CD34 cells were 2├Ś108/kg (range 0.3-12.3 ├Ś108/kg) and 11├Ś106/kg (range 4.6-23.2 ├Ś106/kg), respectively. In the 2nd ASCT, those values were 1.6├Ś108/kg (range 0.3-4.1 ├Ś108/kg) and 7.2├Ś106/kg (range 3.3-23.5├Ś106/kg), respectively. All patients achieved successful engraftments.
In the 1st ASCT, median time for ANC to exceed 500/mm3 and platelet count to exceed 20,000/mm3 was 10 days (range, 9-26 days) and 13.5 days (range, 7-24 days), respectively. In the 2nd ASCT, those values were 11 days (range 8-25 days) and 12 days (range, 9-34 days), respectively. The 2nd ASCT was performed within 1 year after the 1st ASCT in 11 patients (median 4.4 months, range 2.1-8.9 months), and one patient had 4 year interval.
Table 4 shows the response at each step of the therapy. After induction chemotherapy, two patients (16.7%) experienced a CR. After the 1st ASCT, seven patients (58.3%) experienced a CR. And after the 2nd ASCT, eight patients (67%) experienced a CR. The overall response rate was 91%. After the 2nd ASCT, four patients from the favorable group experienced a CR and the remaining patients experienced a PR. Four patients from the unfavorable group experienced a CR, one patient experienced a SD and one patient was not available due to treatment-related mortality.
The median follow up for the nine surviving patients was 18.2 months (range, 8.4-50.4). The median duration of OS was not reached and 59% of the patients reached the 3 year estimated OS. The median of EFS was 25.6 months (95% CI, 10-41) and 40% of the patients reached the 3 year EFS (Figure 1). Three patients who were included in the unfavorable group died. Of the three deaths, two were attributed to MM and one to infection.
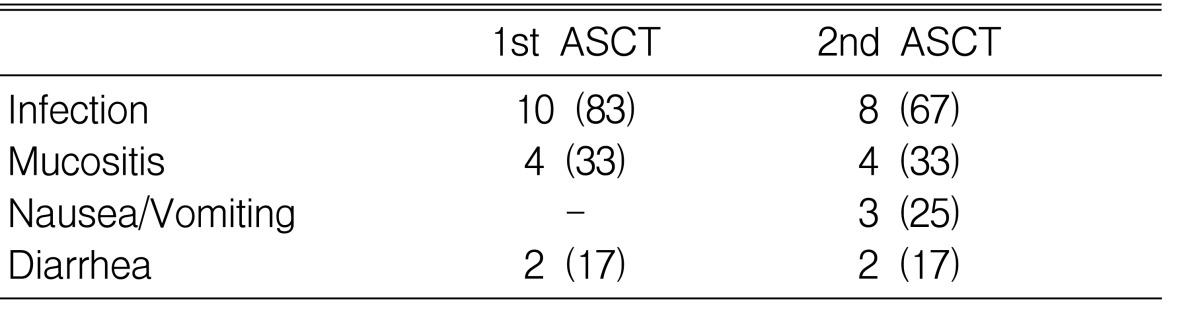
Table 5 summarizes the side effects occurring during HDT with ASCT. The most frequent, non-hematologic side effects were infection, nausea/vomiting, mucositis and diarrhea. During the course of HDT, in 10 patients developed fever during the 1st ASCT and in 12 the 2nd ASCT. The median duration of intravenous antibiotics administration was 11 days (range, 6-18 days) and 8.5 days (range, 5-33 days) during the 1st and 2nd ASCT, respectively. Only one patient experienced grade 4 toxicity (treatment-related infection) during ASCT.
This is the first report describing double ASCT in Korean MM patients. Conventional therapy for MM remains ineffective, with few complete responses3). However, recently two prospective randomized trials demonstrated that HDT with ASCT is superior to conventional therapy in terms of RR, EFS and OS7, 8). VAD induction chemotherapy was used because of its non-cross resistance to melphalan. In one study, the overall response rate and CR rate after VAD were 25% and 91%, respectively. These results were similar to other studies12, 13).
HDT with ASCT is safe, with associated treatment related mortality less than 5%, and effective consolidation therapy for patients responding to initial induction therapy14). A 30~50% CR may be achieved with this approach in newly diagnosed MM, and tumor burden reduction may be converted into prolongation and survival7, 15-17). However, most patients with MM are not cured by a single course of HDT, and eradication of residual Myeloma cells remains a major challenge18). New strategies to monitor and control minimal residual disease following ASCT are necessary. None of the parameters affected EFS and OS due to the small number of patients19, 20). Maintenance chemotherapy, thalidomide, proteosome inhibitors, bisphosphonates, immune therapy (idiotypic or DNA vaccination, vaccination with idiotype pulsed dendritic cells) and double ASCT are all currently being evaluated21-24).
The impact of double transplants was well documented in the largest clinical trial13). In 231 of newly diagnosed patients, the CR rate increased from 26% after the 1st ASCT to 41% after the 2nd ASCT. Two successive ASCT, each preceded by HDT, improved overall survival among patients with MM more than a single ASCT after HDT did9). In the present study, the CR rate increased from 58.3% after the 1st ASCT to 67% after the 2nd ASCT. However, it was difficult to determine whether the results were analyzed exactly. We were unable to compare with a single and double ASCT due to the short follow up and the relatively small number of patients in the study.
Superior EFS and OS were associated with the absence of unfavorable karyotype (11q breakpoints and/or 13 deletion) and low ╬▓2 microglobulin levels13). In the French myeloma study, chromosome 13 abnormalities by FISH, ╬▓2 microglobulin, and Ig A isotype produced a very powerful staging system with respect to HDT25). Another study reported that ╬▓2 microglobulin, LDH, age and treatment assignment were significantly related to survival9).
In this study, we were unable to determine the prognostic factors associated with survival due to small number of patients in the study. Moreover, we performed cytogenetic analysis on only three patients; one patient with normal karyotype and two patients with abnormalities (Table 2). Additionally, we compared double ASCT with single ASCT that were performed at the same period and center. In the single transplant, the median follow up was 16.7 months (range, 5.5-109.7 months) for the 45 surviving patients. The median duration of EFS and OS were 23.7 months (95% CI, 14.5-33), 34.1 months (95% CI, 27.8-40.3), respectively. Of the 24 deaths in this group, 18 were attributed to MM, three to infection, one to bone marrow aplasia, one to suicide, one to unknown causes. Compared with single ASCT, EFS (p=0.26) and OS (p=0.34) of double ASCT were not statistically significant due to the short follow up and the relatively small number of patients in the study.
Several potential limitations need to be addressed in this study. First, this was a retrospective study of a single center. Second, the number of patients in the study was very small. As such we are limited in our ability to generalize the results, and therefore were unable to define the prognostic factors associated with survival and CR rate. Third, as a result of clinical and socioeconomic situations the time interval from the start of 1st ASCT to 2nd ASCT was not constant among patients. These obstacles were mainly a result of the Korean medical insurance system, which does not reimburse the patient for the 2nd ASCT procedure. All 12 patients in this report paid for the 2nd ASCT themselves. Despite these limitations, this is the first report showing the outcome of double ASCT for in Korean MM patient.
References
1. Central Cancer Registry Center and Ministry of Health and Welfare. Annual report of the central registry in Korea. 1999.
2. Yang SH, Kim TY, Kim BK, Ko YW, Kwak DK, Kim NK, Kim SH, Kim SR. A statistical study of multiple myeloma in Korea. Korean J Hematol 1995;30:345ŌĆō361.
3. Barlogie B. In: Kipps T, ed. Plasma cell myeloma. William's hematology. 1995;5th ed. Baltimore: McGrawl-Hill, 1109.
4. Boccadoro M, Marmont F, Tribalto M, Avvisati G, Andriani A, Barbui T, Cantonetti M, Carotenuto M, Comotti B, Dammacco F. Multiple myeloma: VMCP/BAP alternating combination chemotherapy is not superior to melphalan prednisolone even in high-risk patients. J Clin Oncol 1991;9:444ŌĆō448PMID : 1999714.

5. McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma cell leukemia and myeloma. Lancet 1983;2:822ŌĆō824PMID : 6137651.


6. Barlogie B, Alexanian R, Dicke KA, Zagars G, Spitzer G, Jagannath S, Horwitz L. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood 1987;70:869ŌĆō872PMID : 3304465.

7. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah H, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 1996;335:91ŌĆō97PMID : 8649495.


8. Barlogie B, Jagannath S, Vesole DH, Naucke S, Cheson B, Mattox S, Bracy D, Salmon S, Jacobson J, Crowley J, Tricort G. Superiority of tandem autologous transplantation over standard therapy of previously untreated multiple myeloma. Blood 1997;89:789ŌĆō793PMID : 9028309.

9. Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 2003;349:2495ŌĆō2502PMID : 14695409.


10. Lee JH, Bang S, Lee S, Kim HS, Ahn JS, Cho EK, Lee JA, Ahn MJ, Jo DY, Kim TY, Park YS, Yoon SS, Lee HB, Suh C, Seong CM, Lee SN, Yoon HJ, Kim S, Kim CS, Park S, Cho KS, Kim BK, Kim HC, Park CH, Kim SH. High dose chemotherapy with autologous stem cell transplantation in multiple myeloma. Korean J Hematol 1999;34:306ŌĆō316.
11. Jacobson JL, Hussein MA, Barlogie B, Durie BG, Crowley JJ. A new staging system for multiple myeloma patients based on the Swothwest Oncology Group (SWOG) experience. Br J Haematol 2003;122:441ŌĆō450PMID : 12877671.


12. Samson D, Gaminara E, Newland A, van de Pette J, Kearney J, McCarthy D, Joyner M, Aston L, Mitchell T, Hamon M, Barett AJ, Evans M. Infusion of vincristine and doxorubicin with oral dexamethasone as first-line therapy for multiple myeloma. Lancet 1989;2:882ŌĆō885PMID : 2571813.


13. Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, Tricot G, Munshi N, Fassas A, Singhal S, Mehta J, Anaissie E, Dhodapkar D, Naucke S, Cromer J, Sawyer J, Epstein J, Spoon D, Ayers D, Cheson B, Crowley J. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 1999;93:55ŌĆō65PMID : 9864146.

14. Harousseau JL, Attal M, Divine M, Marit G, Leblon V, Stoppa AM, Bourhis JH, Caillot D, Boasson M, Abgrall JF. Autologous stem cell transplantation after first remission induction treatment in multiple myeloma: a report of the French Registry on autologous transplantation in multiple myeloma. Blood 1995;85:3077ŌĆō3085PMID : 7756641.


15. Cunningham D, Paz-Ares L, Milan S, Powles R, Nicolson M, Hickish T, Selby P, Treleavan J, Viner C, Malpas J. High dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma. J Clin Oncol 1994;12:759ŌĆō763PMID : 8151319.

16. Fermand JP, Chevret S, Ravaud P, Divine M, Leblond V, Dreyfus F, Meriette X, Broute JC. High dose chemotherapy and autologous blood stem cell transplantation in multiple myeloma: results of a phase II trial involving 63 patients. Blood 1993;82:2005ŌĆō2009PMID : 8104534.

17. Blade J, San Miguel JF, Fontanillas M, Alcala A, Maldonado J, Gracia Conde J, Conde E, Conzalez-Brito G, Moro MJ, Escudero ML, Trujillo J, Pascual A, Rozman C, Estape J, Montserrat E. Survival of multiple myeloma patients who are potential candidates for early high dose therapy intensification / autotransplantation and who were conventionally treated. J Clin Oncol 1996;14:2167ŌĆō2173PMID : 8683251.

18. Corradini P, Voena C, Astolfi M, Ladetto M, Tarella C, Bocadoro M, Pileri A. High-dose sequential chemotherapy in multiple myeloma: residual tumor cells are detectable in bone marrow and peripheral blood cell harvests and after autografting. Blood 1995;85:1596ŌĆō1602PMID : 7888677.

19. Davies FE, Rawstorn AC, Owen RG, Morgan GJ. Minimal residual disease monitoring in multiple myeloma. Best Pract Res Clin Haematol 2002;15:197ŌĆō222PMID : 11987924.


20. Harousseau JL. In: Broudy VC, Abkowitz JL, Vose JM, Bajus JL, eds. Role of transplantation in myeloma. Hematology. 2002;Washington: American Society of Hematology, 221ŌĆō227.
21. Alexnian R, Weber D, Giralt S, Delasalle K. Consolidation therapy of multiple myeloma with thalidomide-dexamethasone after intensive chemotherapy. Ann Oncol 2002;13:1116ŌĆō1119PMID : 12176792.


22. Massaia M. Idiotype vaccination of myeloma patients after chemotherapy. Acta Oncol 2000;39:807ŌĆō808PMID : 11145437.


23. Liso A, Stokerl-Goldenstein KE, Auffermann-Gretzinger S, Benike CJ, Reichardt V, van Beckhoven A, Rajapaksa R, Enlgemen EG, Bleme KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant 2000;6:621ŌĆō627PMID : 11128812.


24. Kroger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, Fehse B, Tgel F, Wittkowsky G, Kuse R, Zander AR. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood 2002;100:755ŌĆō760PMID : 12130482.


25. Facon T, Avet-Loiseau H, Guillerm G, Moreau P, Genevieve F, Zandecki M, Lai JL, Leleu X, Jouet JP, Bauters F, Harousseau JL, Bataille R, Mary JY. Chromosome 13 abnormalities identified by FISH analysis and serum ╬▓2-microglobulin produce powerful myeloma staging system for patients receving high-dose therapy. Blood 2001;97:1566ŌĆō1571PMID : 11238092.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



