 |
 |
| Korean J Intern Med > Volume 18(4); 2003 > Article |
|
Abstract
Background:
To define the final outcome of large cell carcinomas (LCC) after surgical treatment, the histopathology, clinical features and follow-up results of 28 cases were reviewed.
Methods:
Twenty eight patients, with LCC that underwent a surgical resection between 1986 and 2001, at the Severance Hospital, were retrospectively reviewed. We analyzed clinical data, radiological findings, pathologic findings, treatment modalities, and survival.
Results:
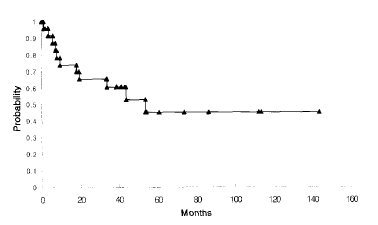
The prevalence of LCC was 2.9% (29 cases) among the surgically resected primary lung cancer cases (1003 cases) during the 15 year period of the study. The mean age of the patients was 59 years old, with 25 male cases. There were 23 smokers, smoking an average of 33 pack years. A cough was the most frequent symptom. There were 15 cases located in the peripheral part of the lung and 26 consisted of a lobulated mass. From a chest CT scan, 26 cases had necrotic portions, which appeared to be low density. The postoperative stages were IA, IB, IIB, IIIA and IV in 1 (3.6%), 11 (39.3%) 8 (28.5%), 7 (25%), 1 case (3.6%), respectively. The concordance rate of the pre- and postoperative stage was 43%. The median survival time and 5 year-survival rate were 54.5 months and 45%, respectively.
A large cell carcinoma (LCC) is one of the typical histologic types of lung cancer, but their incidence is lower than 10%, which is relatively less frequent than other types1). With the use of light microscopy they are defined as large malignant epithelial tumors, with large nuclei, prominent nucleoli and abundant cytoplasm, and they usually have well defined cell borders, without the characteristic features of squamous or small carcinomas or adenocarcinomas2). However, because they are difficult to distinguish from other undifferentiated types, other then by surgical resection, their prevalence varies between 6ŌĆō31% according to reporters, and consequently studies about their definite clinical characteristics and survival rates are lacking3, 4).
With the use of electron microscopy and immunohistochemical staining method, a LCC, with neuroendocrine features, was recently separated as a large cell neuroendocrine carcinoma, with a reported prognosis worse than that of a LCC itself, further augmenting the confusion and debate relating to the diagnosis of LCC5, 6).
Attempts were made to find the concordance rate between the pre- and post-operative diagnoses, clinical characteristics and survival rates of patients diagnosed with a large cell carcinoma, between May 1986 and June 2001, at the Severance Hospital, Yonsei University College of Medicine.
5,069 cases of primary lung cancer were diagnosed, between May 1986 and June 2001, at the Severance Hospital, Medical College, Yonsei University, with 1,003 being surgically resected (Table 1). Of the 31 confirmed LCC cases, 28 were analyzed, as 2 cases, re-diagnosed by pathological re-evaluation as adenocarcinoma and squamous cell carcinoma, and 1 where only an open lung biopsy was performed, were excluded.
For the pathological re-evaluation, the tissue was fixed in 10% formalin, embedded in paraffin, cut into 5-╬╝m sections and stained with Hematoxylin-Eosin. When differentiation of the adenocarcinoma was, Periodic acid-Schiff (PAS) and mucicarmine stain were used, which was classified as a LCC if the results were negative.
Basaloid and large cell neuroendocrine carcinomas especially, were differentiated using neuron-specific enolase (1:100; DAKO, Santa Barbara, CA, USA), chromogranin A (1:10; Boehringer Mannheim, Indianapolis, IN), synaptophysin (1:10; DAKO, Santa Barbara, CA, USA) and CD56 (1:50; DAKO, Santa Barbara, CA, USA).
By reviewing the medical records, sex, age, smoking history, symptom, preoperative diagnosis, findings of initial chest computerized tomography, pre- and post-operative stages, pathological findings, postoperative treatment, the incidence of recurrence, follow-up, and survival rates were sought.
Among the 5,069 primary lung cancer cases diagnosed during the 15 year period, a squamous cell carcinoma was the most common, with 2,230 cases (44%), but there were only 85 cases (1.7%) of LCC. Of the 1,003 surgically resected lung cancer cases, 29 (2.9%) were confirmed as LCC, and 1 case, where only an open lung biopsy was performed, was excluded from the study (Table 1).
The average age of the 28 patients, with LCC, was 59 years, ranging from 41 to 75, with 25 male (average age 59 years) and 3 female (average age 55 years) patients. There were 2 non-smokers and 22 smokers, and 2 cases where this was unknown. The average smoking indices of smokers were 33 pack years (Table 2). Ten patients (36%) had a history of pulmonary tuberculosis, and 3 had previously been treated for extrapulmonary malignant tumors (hepatocellular carcinoma, advanced gastric cancer, and laryngeal cancer). At the time of diagnosis they were completely cured, which had no effect on the treatment and prognosis of lung cancer. A cough was the most common symptom at the time of diagnosis (Table 3).
A fine needle aspiration biopsy was carried out in 14 cases (50%), and was the most common pre-operative diagnostic method. A bronchoscopic biopsy and bronchial washing were the methods of diagnosis in 10 (36%) and 9 (35%) cases, respectively (Table 4). Among the 28 cases, 23 were preoperatively diagnosed with lung cancer. The preoperative histologic cell types of lung cancer were adenocarcinomas, squamous cell carcinomas, unclassified non-small cell cancers, and LCC in 6, 9, 7 and 1 case, respectively (Table 5).
According to the preoperative computerized tomography, the tumors were observed at the center and the periphery in 13 and 15 cases, respectively. The mass was most commonly located at the right lower lobe (9 cases, 32.2%), and the margin was lobulated in 20 cases (71%). In 26 cases (93%) the internal density was low, suggestive of central necrosis.
The postoperative pathological examinations revealed central necrosis in all but one case (96%). If a lymph node was larger than 1cm in diameter it was considered as tumor involved, the sensitivity, specificity and accuracy for detecting mediastinal iymph node metastasis were 100, 52, and 61%, respectively (Table 7).
The concordance rate of the pre- and post-operative stage was 43%, i.e. 12 out of 28 cases; with stages IA, IB, IIB and IIIA in 1, 4, 2 and 5 cases, respectively (Table 8). The complete resection rate was 93% (26 cases). In 2 cases, where the resection margin was involved with the tumor, the tumors recurred at the margin, and the patients expired 55 and 7 months following the operation. A pneumonectomy was the most commonly performed extension of the resection (14 cases, 48%).
Ten patients received chemotherapy as a postoperative adjuvant therapy, but in the recurred cases, chemotherapy was not undertaken due to the poor general condition or refusal of the patients.
Of the 13 cases where postoperative radiotherapy was undertaken, the therapy was adjuvant in 11, and conservative, for brain and bone metastasis, in 2.
At the follow-up to August, 2001 13 patients still survived, with stages IA, IB, IIB, IIIA and IV in 7, 2, 2 and 1 cases, respectively, and there were no recurrences following a complete resection In IA, IB, and IIB (10 cases).
The median follow-up was 41.5 months, ranginge from 3 to 143 months. An extrapulmonary recurrence occurred in 6 cases, with a lymph node recurrence being the most common (4 cases), followed by bone (3 cases), liver (1 case), brain (1 case), maxillary sinus (1 case) and pericardium (1 case). The median survival time and 5-year survival rate of the 28 cases were 54.5 months and 45% (Figure 1). Due to the small number of cases, the prognostic factors could not be analyzed.
According to the 1967 WHO criteria, amended in 1982, a LCC is defined as ŌĆ£a malignant epithelial tumor, with large nuclei, prominent nucleoli and abundant cytoplasm, usually with well defined cell borders, but without the characteristics features of squamous or small cell carcinomas or adenocarcinomasŌĆØ. Undifferentiated tumors, with intracytoplsmic mucin, which had previously been categorized as LCC, have been re-categorized as adenocarcinomas2). Even though immunohistochemical staining and electron microscopy can be used to detect features of differentiation (squamous, glandular, neuroendocrine), the diagnosis of a LCC is mainly based on routine histochemical staining methods and the light microscopic findly2). Even with these diagnostic criteria, it is not easy to distinguish a poorly differentiated LCC from a poorly differentiated squamous cell carcinoma or adenocarcinoma.
Recently, Travis et al5) suggested that a non-small cell carcinoma of the lung, viewed as a neuroendocrine differentiation by light microscopy, and confirmed by immunohistochemical stain and electron microscopy, but not categorized as another neuroendocrine tumor, such as carcinoid, atypical carcinoid, and small cell carcinoma, should be named a large cell neuroendocrine carcinoma, on the basis of the reports suggesting such tumors have a poor prognosis. The WHO/IASLC lung cancer classification of 1999 defined a large cell neuroendocrine carcinoma as a variant of a LCC7).
Our relative prevalence of a LCC was 1.7%, 85 out of 5,069 lung cancers, which was lower than the reports of other Countries, including one by Travis et al. that reported a prevalence of about 10%1, 3, 4), which was also lower than the 6.3% prevalence reported by Kim et al8). Even if only the surgically resected primary lung cancers were considered, the 2.9% prevalence was still lower than the 3.5 and 7.6% reported by Ham et al9) and Han et al10). Our suggestions for this lower prevalence, compared to previous reports, are as followed; firstly, since 1982 some LCC were reclassified as mucinous adenocarcinoma. Secondly, if not surgically resected, a LCC could have been misdiagnosed as another type due to central necrosis and hemorrhage. A report by Kim et al.8) analyzed the primary lung cancers that occurred between 1957 and 1977, and at that time the prevalence of an adenocarcinoma was 11.9%. Our report dealt with the lung cancers that occurred between 1986 and 2001, and while the prevalence of adenocarcinomas have increased to 27% since the mid-1980s, undifferentiated cancers, including LCC, decreased, possibly due to the wider application of immunohistochemical staining methods.
Table 2 summarizes the clinical characteristics of our patients. The gender ratio was 8.3:1, with a male predominance, which was similar to the 10.5:1 ratio reported by Han et al10), but different from the 3.9:1 reported by Kim et al8), which was based on all lung cancers. Travis et al reported a 2.2:1 ratio5).
The average smoke index of the 22 smokers was 33 pack years. Of the 25 male patients, 3 were non-smokers, 21 (88%) had a positive history of smoking and 1 had an unknown history. Compared to the study of Barbone et al11), where 95% of the male patients with a LCC were reportedly smokers, ours showed a slightly lower rate. It is unknown whether smoking was associated with the predominance of LCC prevalence in male patients, and to find other involved factors involved more studies will be required.
Our study revealed that malignant cells were found in only 1 out of 25 cases using the sputum cytology method; with a diagnostic rate was 4%. According to Gledhill et al.12) the low diagnostic rate of the sputum cytology method was due to a too smaller amount of sputum and number of diagnostic trials. To overcome this short fall, he suggested that an adequate amount of sputum should be acquired, in the morning, by coughing after a deep breath, and the tests should be repeated more than 4 times. In our study, the low diagnostic rates, even with 13 cases of central tumor and 11 cases of hemorrhage, were thought be due to the acquisition of sputum from just one test and the inaccuracy of the collection method. This emphasizes the need for repeated and accurate sputum cytology tests.
Only 10 out of 26 cases (38%) were diagnosed as lung cancer using fiberoptic bronchoscopy, with a diagnostic rate of 38%. The low diagnostic rate was because in 7 cases, where the lesion was not visible, all the biopsy samples were negative, with only 5 out of 10 cases having visible lesions, and in 5 out of 9 cases where a transbronchial biopsy was performed, was lung cancer diagnosed. Even the diagnostic rate of the visible lesion was low, as severe necrosis and hemorrhage hindered the diagnosis. Including the 2 cases where the bronchial washing cytology test was positive for malignancy, the diagnostic rate by bronchoscopy was 46.2%, i.e. 12 out of 26 cases. According to Ham et al.9) in 189 cases where bronchoscopy was performed, the diagnostic rate was 71.4%, and according to Kamholz et al.13) this value was 73%. Mak et al.14) also reported on 125 cases where the tumor was visible with bronchoscopy, where the positive rate offor a biopsy was 76%, of the bronchial washing method was 49.6%, and of the bronchial brushing method was 52%. In 63 cases where the tumor was not visible, the corresponding figures were 36.5, 38.1, and 28.6%, respectively. From this it was recommended combining the washing and brushing methods with a biopsy during bronchoscopy. In our study, the malignancy detection rate of the bronchial washing method was 36%, 9 out of 26 cases, which was slightly lower than the reports by Mak et al14). Even when the tumor was visible, a biopsy through bronchoscopy might be unable to diagnose, due to too few, or no, cancer cells in the tumor, or from tissue damage that could cause difficulty in the interpretation. Especially in LCC, central necrosis, hemorrhage and obstructive pneumonia might be anticipated in the low diagnostic rate.
The diagnostic rate of a fine needle aspiration biopsy was 88%, i.e. 14 out of 16 cases. According to Allison et al15), the diagnostic rate was 90%, with almost no false positive results, and a false negative rate of 5ŌĆō10%, which were compatible to our results.
Among 28 surgically resected cases, only 1 was preoperatively diagnosed as a LCC. Confusion in the diagnostic guide could be the reason for the low preoperative diagnostic rate, with central necrosis, hemorrhage and obstructive pneumonia also making diagnosis potentially difficult by methods other than surgery. For precise confirmation, an analysis of the concordance rate between preoperative diagnosis of a LCC and the postoperative confirmation of the same histologic type is required. In our literature review no such study could be found.
The tumor was sited at the periphery in 15 cases (54%), with a similar number being found centrally. Taking into account all the LCC, the peripheral type might be more abundant our study only considered the patients having undergone surgery. The tumors were located in the right lung in 18 cases (68%), which was twice as often as in the left lung. According to the report by Kim et al8), the prevalence in the right lung was also twice that in the left lung, but reasons for this was unknown. Radiologically, our study showed that surgically confirmed LCC were distributed evenly at the periphery and center, with lobulated rather than smooth margins, with the presence of central necrosis.
Computerized tomography is most useful for diagnosing the extension of a primary tumor, its relationship to adjacent organs, mediastinal lymph node enlargement and also helps to determine the resectability. However, according to Heidenberg et al16), the size of lymph node alone could not differentiate metastasis from inflammation, as not all the enlarged lymph nodes were involved with the cancer, where 87% of the involved lymph nodes were larger than 1.4 cm. With the criterion of 1.5 cm, Modini et al17) achieved sensitivity, specificity and accuracy of 55, 91 and 75%, and in the report by Ham et al9), were 78.9, 72.7 and 75%, respectively. With the criterion of 1 cm, according to Webb et al18), the sensitivity and specificity were 52 and 69%, respectively, and a large scaled multivariable analysis by Dales et al.19) revealed a sensitivity, specificity and accuracy each of 79%.
Our study showed that if the enlargement of the mediastinal lymph nodes were defined as larger than a diameter of 1 cm, the sensitivity was 100%, but the specificity was 52%. For the parenchymal and the hilar lymph node, the sensitivity was 20%, but the specificity and accuracy were 87 and 75%, respectively, but compared to other studies, the accuracy was still low. Pulmonary tuberculosis, which is highly prevalent in Korea, and obstructive pneumonia and central necrosis by cancer, could induce reactive lymph node hypertrophy. The concordance rate of the pre- and postoperative stage was as low as 43% due to the difference between the pathologic findings and the computerized tomography with regard to the lymph node enlargements and major organ involvements.
Generally, surgical resection is the primary treatment for a non-small cell carcinoma, including a LCC, where after radiotherapy or chemotherapy is undertaken according to the stage. Mitchell et al.20) analyzed the prognosis of 208 patients with a LCC, and reported that the median survival time and 5-year survival rate of 55 patients that received surgical treatment were 13 months and 21.2%, and for 4 patients that received postoperative radiotherapy were 6.2 months and 0%. Downey et al.21) reported that even with precise surgical staging and clear diagnostic criteria, the prognosis of LCC was poor, regardless of the treatment. At the time of diagnosis, 90% of cases were already at an advanced stage (stage III, IV), with only 10% at stage I. He reported that most patients expired within 9 monthsŌĆÖ with an average survival time for the stage I patients of 15.9 months and therefore the prognosis was very poor.
According to Downey et al22), the 5-year survival rate of 61 patients with LCC having undergone surgery was 37%, suggesting an improvementd in the results due to aggressive surgery. The 28 patients in our study had a median survival time and 5-year survival time of 54.5 months and 45%, respectively, which were higher than reports from other Countries. Compared to the report of Mitchell et al20), the distribution of stages were similar, but those of the postoperative chemotherapy and radiotherapy were not, and compared to the reports of Downey et al21, 22), more low stages, such as stages I and II, were included in our study (Table 9). According to a Korean report by Lee et al23), in 1987, the 3-year survival rate of 8 patients with LCC out of 147 patients with primary lung cancer that underwent a surgical resection was 44.5%, and in the report of Han et al10), in 1996, the 2-year survival rate of 29 out of 382 such patients was 59%.
There have been few trials of postoperative chemotherapy and radiotherapy for LCC, suggesting multi-centered clinical studies, based on a larger population of patients, are needed.
Table┬Ā1.
Frequency of primary and surgically resected primary lung cancers, by histologic types, at the Severance Hospital during the 15 year period (1986ŌĆō2001)
Table┬Ā2.
Clinical features of large cell carcinomas, 28 cases
| Case | Sex | Age | Smoking (PY) |
FOB/TBLB
|
FNAB | Preop-pathol | Preop-staging | Postop-staging | Op-name | CTx | RTx | Follow up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bx | Cyto | ||||||||||||
| 1 | M | 45 | 20 | N | N | N | N | T1N2M0 (IIIA) | T1N2M0 (IIIA) | MR | adj | adj | Alive (74 mon) |
| 2 | M | 41 | 0 | TŌłÆ | ŌłÆ | ŌłÆ | ŌłÆ | T2N2M0 (IIIA) | T2N2M0 (IIIA) | P | adj | adj | Alive (45 mon) |
| 3 | M | 67 | 60 | F+ | + | N | Squam | T2N1M0 (IIB) | T2N0M0 (IB) | P | N | N | Alive (86 mon) |
| 4 | M | 57 | 40 | FŌłÆ | + | + | Squam | T2N2M0 (IIIA) | T2N0M0 (IB) | P | N | N | Alive (101 mon) |
| 5 | M | 61 | 30 | F+ | + | ŌłÆ | NSCLC | T3N1M0 (IIIA) | T2N1M0 (IIB) | P | adj | adj | Alive (939 mon) |
| 6 | M | 44 | 30 | TŌłÆ | ŌłÆ | N | ŌłÆ | T2N0M0 (IB) | T2N0M0 (IB) | L | adj | adj | Alive (42 mon) |
| 7 | M | 52 | 45 | F+ | ŌłÆ | N | Adeno | T2N2M0 (IIIA) | T2N0M0 (IB) | BL | adj | N | Alive (114 mon) |
| 8 | M | 57 | 40 | FŌłÆ | ŌłÆ | + | NSCLC | T1N0M0 (IA) | T1N0M0 (IA) | P | N | N | Alive (61 mon) |
| 9 | M | 64 | 45 | TŌłÆ | ŌłÆ | + | Squam | T2N1M0 (IIB) | T2N0M0 (IB) | L | N | N | Alive (3 mon) |
| 10 | M | 68 | 0 | T+ | + | N | Adeno | T2N0M0 (IB) | T2N1M0 (IIB) | L | adj | adj | Died* (66 mon) |
| 11 | M | 64 | 0 | F+ | + | N | Squam | T4N2M0 (IIIB) | T3N0M0 (IIB) | P | N | adj | Died* (55 mon) |
| 12 | M | 72 | 50 | FŌłÆ | ŌłÆ | + | Adeno | T3N2M0 (IIIA) | T2N2M0 (IIIA) | L | adj | adj | Died* (55 mon) |
| 13 | M | 59 | 50 | FŌłÆ | ŌłÆ | + | Squam | T4N2M0 (IIIB) | T3N1M0 (IIIA) | P | adj | a+p | Died* (20 mon) |
| 14 | M | 75 | 30 | T+ | ŌłÆ | + | Squam | T3N0M0 (IIB) | T3N0M0 (IIB) | L | N | N | Died* (7 mon) |
| 15 | M | 72 | 50 | N | N | + | Squam | T3N0M0 (IIB) | T3N0M0 (IIB) | L | N | adj | Died* (7 mon) |
| 16 | M | 70 | 25 | FŌłÆ | ŌłÆ | + | Adeno | T2N0M0 (IB) | T2N0M0 (IB) | L | N | N | Died* (1 mon) |
| 17 | F | 61 | 7 | TŌłÆ | + | + | NSCLC | T2N2M0 (IIIA) | T2N2M0 (IIIA) | P | N | N | Died* (29 mon) |
| 18 | F | 42 | 0 | T+ | + | N | Adeno | T2N2M0 (IIIA) | T2N0M0 (IB) | BL | N | N | Alive (35 mon) |
| 19 | M | 62 | 60 | FŌłÆ | ŌłÆ | N | ŌłÆ | T4N3M0 (IIIB) | T3N0M0 (IIB) | P | N | adj | Alive (41 mon) |
| 20 | M | 43 | NR | FŌłÆ | ŌłÆ | + | NSCLC | T4N2M0 (IIIB) | T2N0M0 (IB) | L | N | pall | Died* (20 mon) |
| 21 | M | 56 | 40 | FŌłÆ | ŌłÆ | + | Squam | T4N2M0 (IIIB) | T4N2M1 (IV) | P | adj | N | Alive (49 mon) |
| 22 | M | 58 | 40 | FŌłÆ | ŌłÆ | + | NSCLC | T3N2M0 (IIIA) | T3N1M0 (IIIA) | P | adj | N | Died* (7 mon) |
| 23 | M | 64 | 40 | FŌłÆ | ŌłÆ | + | Squam | T4N0M0 (IIIB) | T3N1M0 (IIIA) | P | N | adj | Died* (34 mon) |
| 24 | M | 65 | 45 | T+ | + | N | NSCLC | T2N2M0 (IIIA) | T2N0M0 (IB) | P | N | N | F/U loss |
| 25 | F | 63 | NR | F+ | ŌłÆ | N | Adeno | T3N2M0 (IIIA) | T3N0M0 (IIB) | BL | N | N | F/U loss |
| 26 | M | 56 | 40 | FŌłÆ | ŌłÆ | + | NSCLC | T2N0M0 (IB) | T2N0M0 (IB) | L | N | N | F/U loss |
| 27 | M | 63 | 30 | FŌłÆ | ŌłÆ | N | ŌłÆ | T4N2M0 (IIIB) | T3N0M0 (IIB) | L | N | N | F/U loss |
| 28 | M | 60 | 40 | T+ | + | N | Lcca | T2N0M0 (IB) | T2N0M0 (IB) | P | N | N | Alive (143 mon) |
Abbreviations: Adeno, adenocarcinoma; adj, adjuvant; a+p, adjuvant + palliative; BL, bilobectomy; Bx, biopsy; CTx, chemotherapy; Cyto, washing cytology; FNAB, fine needle aspiration biopsy; FOB (F), fiberoptic bronchoscopy; L, lobectomy; Lcca, large cell carcinoma; mon, months; MR, mass resection; N, not performed; NSCLC, non-small cell lung carcinoma; Op, operation; P, pneumonectomy; pall, palliative; PY, pack year; RTx, radiation therapy; Squam, squamous cell carcinoma; TBLB (T), transbronchial luhg biopsy;
Table┬Ā3.
Clinical Symptoms
| Symptoms | No. of patients |
|---|---|
| Cough | 19 (68.0%) |
| Sputum | 15 (54.0%) |
| Chest pain | 11 (39.0%) |
| Hemoptysis | 11 (39.0%) |
| Dyspnea | 5 (18.0%) |
| Weight loss | 5 (18.0%) |
| Asymptomatic | 2 (7.0%) |
| Chest wall mass | 1 (3.5%) |
Table┬Ā4.
Malignancy rate of preoperative diagnostic methods
| Methods | No. of patients | Malignancy diagnosis rate |
|---|---|---|
| Fine needle aspiration biopsy | 16 | 14 (88%)* |
| Fiberoptic bronchoscopy | 26 | 10 (38%) |
| - Visible mass | 10 | 5 (50%) |
| - No visible mass | 7 | 0 (0%) |
| - TBLB | 9 | 5 (55%)* |
| Bronchial washing | 26 | 9 (35%) |
| Non-diagnostic | 5 | 0 (0%) |
Table┬Ā5.
Preoperative diagnostic methods and cell types
|
Cell type
|
FOB | FNAB | Total |
|---|---|---|---|
| Dx methods | |||
| Adenocarcinoma | 4 (40%) | 2 (14%) | 6 (25%) |
| Squamous cell carcinoma | 3 (30%)* | 7 (50%)* | 10 (42%)* |
| NSCLC unknown type | 2 (20%) | 5 (36%) | 7 (29%) |
| Large cell carcinoma | 1 (10%) | 0 (0%) | 1 (4%) |
|
|
|||
| Total | 10 (100%)* | 14 (100%)* | 24 (100%)* |
Table┬Ā6.
Chest CT scan and pathologic findings in 28 cases of a large cell carcinoma
Table┬Ā7.
Comparison between preoperative chest CT scan and postoperative pathology for hilar and mediastinal lymph node infiltration
| Pathology |
Chest CT findings
|
|||
|---|---|---|---|---|
| N1 nodes | N2 nodes | |||
|
|
||||
| (+) | (ŌłÆ) | (+) | (ŌłÆ) | |
| (+) | 1 | 4 | 5 | 0 |
| (ŌłÆ) | 3 | 20 | 11 | 12 |
Table┬Ā8.
Comparision of preoperative and postoperative stages
Table┬Ā9.
Comparison of clinical features and surgical treatment outcomes of large cell carcinomas
| Author (published year) | Mitchel et al20) (1980) | Downey et al21) (1989) | Downey22) (1999) | This study |
|---|---|---|---|---|
| Study durations (year) | 1968 ŌĆō 1978 | 1973 ŌĆō 1983 | 1982 ŌĆō 1986 | 1986 ŌĆō 2001 |
| Subjects | All LCC | All LCC | Surgically resected LCC | Surgically resected LCC |
| Total No. of LCC (% of all lung cancer) | 208 (9.0) | 96 (6.7) | NR | 85 (1.7) |
| Surgically resected cases (% of all LCC) | 59 (28.4) | 13 (13.3) | 61 (NR) | 28 (32.9) |
| Centrality of mass (%) | Peripheral (43) | NR | NR | Peripheral (54) |
| Smoker (%) | 95 | 96 | 98 | 85 |
| Most common Symptom (%) | Cough (61.5) | Cough (22.0) | Cough (38.0) | Cough (68.0) |
| FOB biopsy Dx rate (%) | 87/208 (42) | 35/96 (37) | 23/42 (55) | 10/28 (36) |
| Sputum cytology Dx rate (%) | 29/208 (14) | NR | 4/31 (13) | 1/25 (4) |
| Postoperative stage (%) | I, II 46 (78.0) | I 6 (46.1) | I, II 37 (61.0) | I, II 20 (71.4) |
| III 13 (22.0) | III 5 (38.5) | III 22 (36.0) | III 7 (25.0) | |
| IV 2 (15.4) | IV 2 (3.0) | IV 1 (3.6) | ||
| Surgery and other treatment modality (%) | Sur 55 (93.2) | Sur 9 (69.2) | NR | Sur 13 (46.4) |
| Sur+RTx 4 (6.8) | Sur+RTx 4 (30.8) | Sur+RTx 5 (17.9) | ||
| Sur+CTx 3 (10.7) | ||||
| Sur+CTx+RTx 7 (25.0) | ||||
| Median survival of surgically resected patients (months) | 13.0 | Overall 15.9 | NR | 54.5 |
| 5-year survival of surgically resected patients (%) | 21.2 | Unable | NR | 45.0 |
REFERENCES
2. World Health Organization. The World Health Organization histological typing of lung tumors: second edition. Am J Clin Pathol 77:123ŌĆō1361982.


3. Kirsh MM, Rotman H, Argenta L, Bone E, Cimmino V, Tashian J, Ferguson P, Sloan H. Carcinoma of the lung: results of treatment over ten years. Ann Thorac Surg 21:371ŌĆō3771976.


4. Delmonte VC, Alberti O, Saldiva PH. Large cell carcinoma of the lung Ultrastructural and immunohistochemical features. Chest 90:524ŌĆō5271986.


5. Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 15:529ŌĆō5531991.


6. Wick MR, Berg LC, Hertz MI. Large cell carcinoma of the lung with neuroendocrine differentiation. A comparision with large cell undifferentiated pulmonary tumors. Am J Clin Pathol 97:796ŌĆō8051992.


7. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Collaborators from 14 Countries. Histological typing of lung and pleural tumors. Berlin: Spinger Verlag, 1999.
8. Kim Chang Jin, Park Chan II, Ro Jae Yun, Choi In Joon, Lee Yoo Bock. Clinico-pathological studies on primary carcinoma of the lung among Koreans. Korean J Pathol 13:451ŌĆō4611979.
9. Hahm Shee Young, Sung Sook Whan, Kim Joo Hyun. Long-term results of surgical treatment for primary lung cancer. Korean J Thoracic & Cardiovasc Surg 20:730ŌĆō7441987.
10. Han Hye Seung, Seo Jeong-Wook, Ham Eui Keun. Immunohistochemical study of primary large cell undifferentiated carcinoma of the lung. Korean J Pathol 30:417ŌĆō4261996.
11. Barbone F, Bovenzi M, Cavallieri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest 112:1474ŌĆō14791997.


12. Gledhill A, Bates C, Henderson D, DaCosta P, Thomas G. Sputum cytology: a limited role. J Clin Pathol 50:566ŌĆō5681997.



14. Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fiberoptic bronchoscopy in the diagnosis of lung cancer. Thorax 45:373ŌĆō3761990.



15. Allison DJ, Hemingway AP. Percutaneous needle biopsy of the lung. Br Med J Clin Res Ed 282:875ŌĆō8781981.



16. Rea HH, Shevland JE, House AJ. Accuracy of computed tomographic scanning in assessment of the mediastinum in bronchial carcinoma. J Thorac Cardiovasc Surg 81:825ŌĆō8291981.


17. Modini C, Passariello R, Lascone C, Cicconetti F, Simonetti G, Zerilli M, Tirindelli-Danesi D, Stipa S. TNM staging in lung cancer: role of computed tomography. J Thorac Cardiovasc Surg 84:569ŌĆō5741982.


18. Webb WR, Gatsonis C, Zerhouni EA, Heelan RT, Glazer GM, Francis IR, McNeil BJ. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178:705ŌĆō7131991.


19. Dales RE, Stark RM, Raman S. Computed tomography to stage lung cancer. Approaching a controversy using meta-analysis. Am Rev Respir Dis 141:1096ŌĆō11011990.


20. Mitchell DM, Morgan PG, Ball JB. Prognostic features of large cell anaplastic carcinoma of the bronchus. Thorax 35:118ŌĆō221980.



21. Downey RS, Sewell CW, Mansour KA. Large cell carcinoma of the lung: a highly aggressive tumor with dismal prognosis. Ann Thorac Surg 47:806ŌĆō8081989.


22. Downey RJ, Asakura S, Deschamps C, Colby TV. Large cell carcinoma of the lung: results of resection for a cure. J Thorac Cardiovasc Surg 117:599ŌĆō6041999.


23. Lee Doo Yun, Kim Hae Kyoon, Hong Sung Nok, Cho Bum Koo, Kim Sung Kook, Kim Joo Hang. Long term results of surgical treatment of lung carcinoma. J Thorac Cardiovasc Surg 20:328ŌĆō3411987.
-
METRICS

- Related articles
-
Clinical Features of Waldenstrom Macroglobulinemia in Korea2004 September;19(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


