 |
 |
| Korean J Intern Med > Volume 20(1); 2005 > Article |
|
Abstract
Ileovesical fistula is a very rare clinical entity, the most frequent cause of which is Crohn's disease. Furthermore, it is an exceptionally rare complication of malignancies. We experienced one case of ileovesical fistula which had been caused by hepatocellular carcinoma (HCC) arising from the noncirrhotic liver.
A 27-year-old man was diagnosed with HCC in a noncirrhotic liver. Despite treatment with transarterial chemoembolization (TACE), the disease status became more aggravated. The patient complained of dysuria, fecaluria, and intermittent lower abdominal pain. Pelvic CT scan showed a soft tissue mass of 6 cm abutting on the distal ileum which was downwardly displaced. Barium study of the small bowel showed a fistula between the small bowel loop and the urinary bladder. Upon operation, adhesion and fistula were found between the ileum and the urinary bladder. The microscopic findings of the surgical specimen were compatible with metastatic HCC. We confirmed that ileovesical fistula had been caused by metastatic HCC.
Clinically, ileovesical fistulas are very rare entities. The incidence of vesicoenteric fistulas has been reported to occur in 0.01% to 0.05% of surgical admissions1). In one study of 56 instances of vesicoenteric fistulas, ileovesical fistula was found in only 3 patients (5.3%)2).
Hepatocellular carcinomas (HCCs) account for 90% of primary liver cancers; up to 80% of HCCs occur in Korea occur in patients with cirrhotic livers. According to our literature review, several malignancies have been reported as causes of ileovesical fistula, though such complications have not yet been reported to be caused by HCC.
Here, we report a case of ileovesical fistula caused by HCC arising from the noncirrhotic liver.
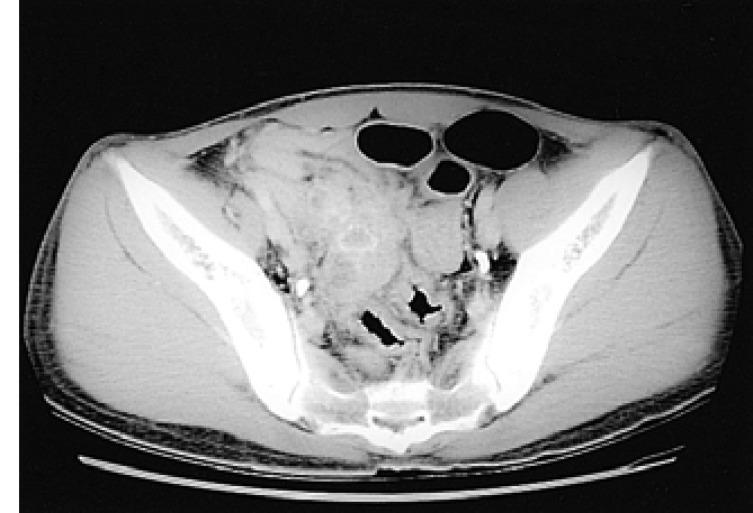
A 27-year-old man was referred to our hospital for the evaluation of hepatic masses. He had consumed 30.0 g alcohol twice weekly for 8 years. On presentation, his vital signs were stable and the physical examination was normal. Liver function test was nonspecific. Viral marker study showed that HBs antigen was negative, anti-HBs antibody positive, and anti-HCV negative. ╬▒-fetoprotein (╬▒FP) was over 50,000 IU/mL and carcinoembryonic antigen (CEA) was 4.94 ng/mL. Abdominal computed tomography (CT) with enhancement revealed two heterogeneous hypodense masses in the right hepatic lobe of about 2.4 ├Ś 2 cm and 3.4 ├Ś 4 cm. No cirrhotic change was observed in the liver. Upper intra-abdominal lymphadenopathy was absent. Liver magnetic resonance imaging (MRI) revealed two relatively well-defined masses in the right hepatic lobe (Figure 1). These lesions were homogeneous low signal intensity on T1-weighted image and slightly inhomogeneous high signal change on T2-weighted image. The tissue diagnosis was obtained by percutaneous biopsy (Figure 2). Immunohistochemical stains were positive for cytokeratin (CK), epithelial membrane antigen (EMA), ╬▒FP, and hepatocyte-specific antigen (HSA). These findings were compatible with HCC. However, evidence of cirrhosis and chronic injury were absent. Under the diagnosis of HCC, he was treated with transarterial chemoembolization (TACE). Abdominal CT scan was performed during the third week after TACE. The scan showed two lipiodol uptake lesions in the right hepatic lobe. Two months later, follow-up CT scan showed a newly developed mass in the caudate lobe about 4.5 ├Ś 4.0 cm in size, with several daughter nodules. A second TACE was performed. Follow-up CT scan after 3 weeks showed defective lipiodol uptake masses in both the right hepatic lobe and the caudate lobe with remnant tumoral enhancement. Thrombus was also observed in the inferior branch of the right portal vein. Several days later, the patient complained of dysuria, fecaluria, and intermittent lower abdominal pain. Pelvic CT scan showed a lobulated soft tissue mass about 6 cm in size (Figure 3). The mass was abutting on
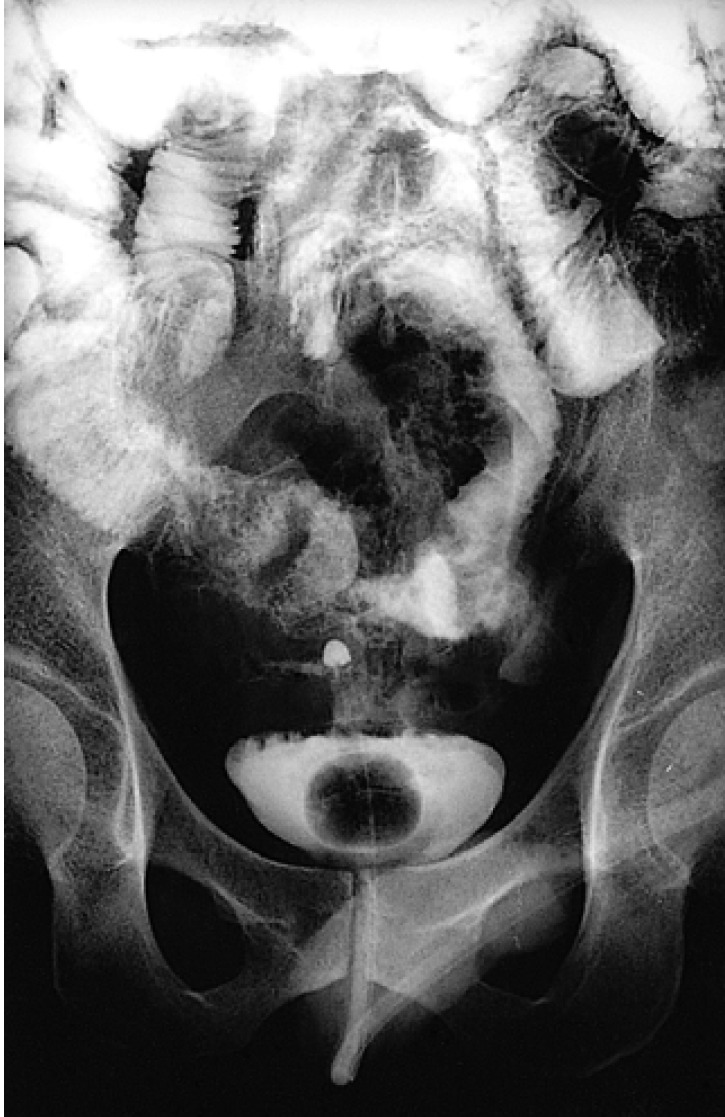
the distal ileum which was downwardly displaced. Barium study of the small bowel showed a fistula between the small bowel loop and the urinary bladder (Figure 4). We diagnosed this as ileovesical fistula and referred him to the surgical unit.
Laparotomy was performed under the diagnosis of ileovesical fistula. There were soft tissue masses between the ileum and the bladder and multiple lymphadenopathies at the mesenteric root. Adhesion and fistula were found between the ileum at 10 cm proximal to the ileocecal valve and the dome of the bladder.
Fistula and soft tissue mass were excised. The distal 65 cm of ileum was segmentally resected and anastomosed end-to-end. The bladder was partially resected and closed. Microscopic examination of the surgical specimen showed similar findings to those of liver biopsy. We confirmed that ileovesical fistula had been caused by implanted intraperitoneal metastases of HCC.
Several malignancies have been reported to be causes of ileovesical fistula. According to our literature review, these include bladder cancer, lymphoid malignancies, and others1, 3-5). Although other various malignancies themselves were not direct causes of ileovesical fistula, radiation enteritis complicated by radiation therapy could also cause the ileovesical fistula6).
In Korea, about 80% of HCCs occur in patients with cirrhotic livers. In our case, HCC occurred in the noncirrhotic liver, and the cause remains uncertain. In one study of 403 patients with HCC, 148 patients (37%) with extrahepatic metastases were identified. The lung, abdominal lymph nodes, and bone are the most common sites of extrahepatic metastatic HCC. Peritoneal metastases were illustrated at CT scan in 16 (11%) of 148 patients with extrahepatic metastatic HCC7). Ho et al. reported that 3% of patients with HCCs in noncirrhotic liver involved the peritoneum8).
Fecaluria, abdominal pain, and pneumaturia were the most common presenting symptoms of vesicoenteric fistulas. Other symptoms were dysuria, gross hematuria, fever and chills, diarrhea, and urine per rectum.
The diagnosis of vesicoenteric fistula can be highly indicated by the above mentioned symptoms, using various diagnostic tests to establish diagnosis. In our particular case, cystoscopic and cystographic examinations showed inflammatory mucosal changes on the dome of the urinary bladder, but could not detect a fistula. Barium study of the small bowel exposed an ileovesical fistula. Kirsh et al. reviewed 1,100 instances of vesicoenteric fistulas reported in the literature9). Cystoscopy revealed either the fistula itself or highly suggestive, localized mucosal abnormalities ("herald lesions") in 86%. The fistula itself was visualized in 39%. Cystography and barium enema demonstrated a vesicoenteric fistula in 32% and 34%, respectively. CT scan revealed abnormalities, such as pelvic masses or bladder or intestinal wall thickening (87%), and revealed a fistula (24%). It should be possible to establish the diagnosis of a vesicoenteric fistula through a combination of cystoscopy, cystography, and barium study in almost all patients. Other diagnostic tests for vesicoenteric fistulas include sigmoidoscopy or colonoscopy, intravenous urography, radioisotope studies, and so on.
Forms of treatment for vesicoenteric fistula include diverting enterostomy, single stage repair, and multi-staged repair. When secondary to radiation necrosis and recurrent tumor, fistulas have an extremely poor outlook with some palliation afforded by a diverting colostomy2). Most authors recommended the single stage repair9). Multi-staged repair is limited to those patients with evidence of intestinal obstruction, uncontrolled local sepsis, and questions of intestinal viability9).
In summary, we experienced one case of ileovesical fistula, which was caused by HCC arising from noncirrhotic liver. Typical symptoms and signs of ileovesical fistula were easily detected. The combination of cystoscopy, cystography, and barium study of the small bowel confirmed the diagnosis of ileovesical fistula, and single stage repair was performed successfully.
References
1. Pugh JI. On the pathology and behaviour of acquired non-traumatic vesico-intestinal fistula. Br J Surg 1964;51:644ŌĆō657PMID : 14222567.


2. Karamchandani MC, West CF Jr. Vesicoenteric fistulas. Am J Surg 1984;147:681ŌĆō683PMID : 6721047.


3. Lalka JP, Novak LI, Tucker JB. Intestinovesical fistula in a patient with chronic lymphocytic leukemia: case report and literature review. Cancer 1982;49:2165ŌĆō2167PMID : 7074531.


4. Kang JG, Hwang CI, Yoon JB. Vesicoileal fistula caused by malignant lymphoma: a case report. Korean J Urol 1984;25:387ŌĆō392.
5. Kato H, Nakamura M, Ito T, Ito Y, Orito E, Ueda R, Tsuzuki T, Mizokami M. Enterovesical fistula complication in B-cell-type lymphoma of the small intestine. J Gastroenterol 2004;39:589ŌĆō591PMID : 15235879.


6. Deitel M, To TB. Major intestinal complications of radiotherapy: management and nutrition. Arch Surg 1987;122:1421ŌĆō1424PMID : 3689119.


7. Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000;216:698ŌĆō703PMID : 10966697.


Figure┬Ā1
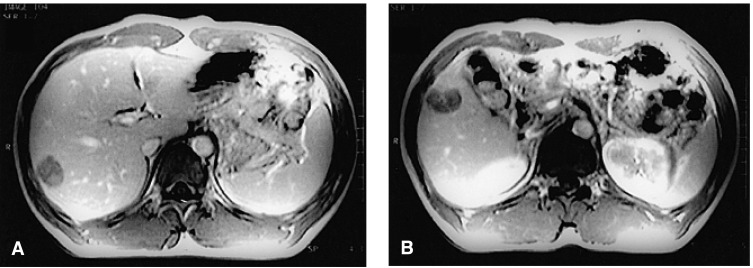
Gadolinium-enhanced dynamic liver MRI, demonstrating two relatively well-defined masses in the right hepatic lobe (A and B). They exhibit central scar-like enhancing structures and peripheral enhancing rims.
Figure┬Ā2
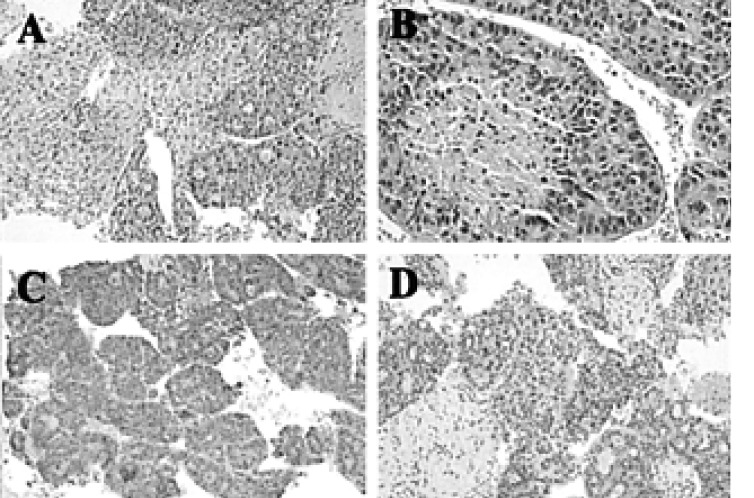
Percutaneous liver biopsy. The submitted specimen shows irregular, thick trabecular pattern of atypical hepatocytes with distinct sinusoidal space (H&E stain, ├Ś100) (A). The tumor cells have hyperchromatic nuclei with coarse chromatin patterns, distinct nucleoli, and abundant eosinophilic cytoplasms (H&E stain, ├Ś200). Immunohistochemical stains are positive for ╬▒FP (C), and hepatocyte-specific antigen (HSA) (D).
-
METRICS

- Related articles
-
Intussusception caused by small bowel metastasis from hepatocellular carcinoma2024 January;39(1)
Newer treatments for advanced hepatocellular carcinoma2014 March;29(2)
Status Quo of Chronic Liver Diseases, Including Hepatocellular Carcinoma, in Mongolia2012 June;27(2)
Nododuodenal Fistula Caused by Tuberculosis2011 December;26(4)
Application of Helical Tomotherapy for Two Cases of Advanced Hepatocellular Carcinoma2011 June;26(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


