INTRODUCTION
Anemia is one of the most common clinical features in patients on long-term hemodialysis. Its etiology is usually multifactorial, but hemolysis is not an infrequent cause1,3). The hemolysis may result from underdialysis, mechanical damage to red blood cells, abnormal erythrocyte metabolism4), overheated dialysate5) or the presence of contaminants in the dialysis bath. The contaminants, such as formaldehyde6), copper7), nitrate8), zinc9) and chloramine10ŌĆō13) in dialysate, have been reported to cause hemolytic anemia and methemoglobinemia.
There was an outbreak of hemolytic anemia in 34 patients on maintenance hemodialysis in Guro Hospital, Korea University Medical Center, during four months from December 1988 to March 1989. A series of studies were tried to identify and neutralize the offending substrates. We detected high levels of chloramine in the treated water. To elucidate the cause of the high chloramine level, we compared the ammonia nitrogen concentrations of our water source (A) with another (B). After we changed the source of the water supply, there were striking improvements in laboratory findings, as well as in the general condition of the patients. In this paper, we report the clinical and laboratory features of the outbreak, the identification of the offending agent, the implementation of control measures and the improved findings after switching the water source.
MATERIALS AND METHODS
1. Description of the Outbreak
From December 1988, we noticed that 34 chronic hemodialysis patients in Guro Hospital, Korea University, suffered from angina or dyspnea more frequently than ever and that there were remarkable decreases in hemoglobin values and increase in reticulocyte counts and transfusion requirements. On peripheral blood smears, we could observe Heinz bodies. After the initial notification of the outbreak, we examined the water treatment system in the hemodialysis unit and reinforced the water treatment by various procedures, such as changing the charcoal column, reverse osmosis system, softner resin and adding ascorbic acid to the tank water for 2 weeks and setting the carbon filter at the bedside unit, but the episodes of hemolytic anemia were not reduced. Finally after we switched the source of the water supply from A into B in April 1989, the hemolysis could be controlled.
2. Laboratory Studies
Transfusion requirements in the hemodialysis unit were counted by the month and by the week around the outbreak. Blood samples from hemodialysis patients were tested for CBC, reticulocyte count, peripheral blood smear (Romanowsky stain and methyl violet vital stain), LDH and ascorbate cyanide test14) before and after the hemolytic episode. Plasma erythropoietin concentrations were determined by radioimmunoassay.
Water from the reverse osmosis system and from the storage tank were analyzed for aluminum, chloramine, copper, nitrate, sulfate and zinc during the outbreak. Levels of total and free residual chlorine from different water sources (A & B) were determined by DPD ferrous titrimetric method15). Routine laboratory data from water filtration plant A and B were reviewed for ammonia nitrogen levels.
RESULTS
1. Episodes of Heinz Body Positive Hemolytic Anemia
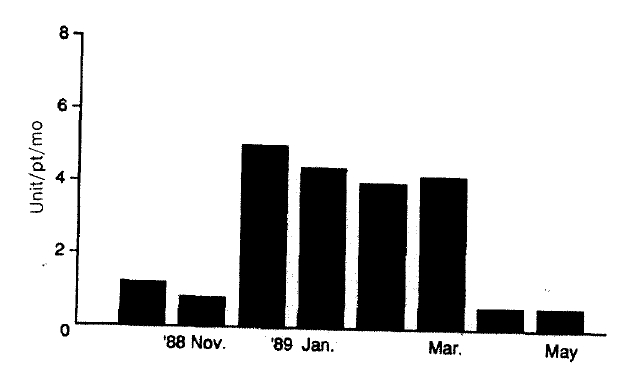
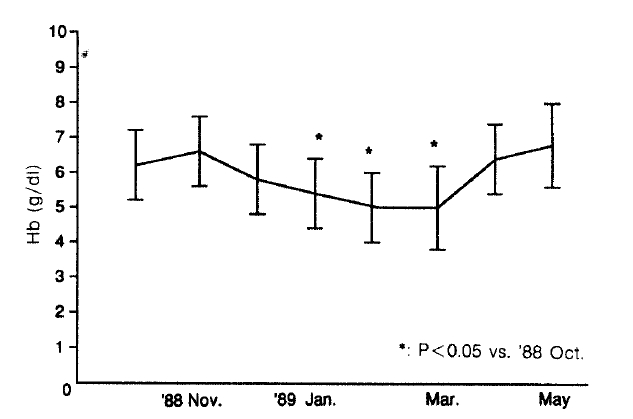
Transfusion requirements increased three times from 1.2 unit/patient in November 1988 to 3.9 units/patients in December 1988 (Fig. 1). In spite of more frequent transfusions, the median hemoglobin values of dialysis patients were barely maintained in the range from 4.81 g/dl to 5.71 g/dl, compared with 6.21 g/dl in the preceding month (p<0.05, vs October 1988) (Fig. 2). Hemolytic anemia developed in 34 of 41 long-term hemodialysis patients (83%). The symptoms of the 34 patients were frequent angina and dyspnea on exertion. In 26 of these patients, Heinz bodies were observed on the peripheral blood smear and the ascorbate-cyanide test was positive in 30 of these patients.
2. Trials to Control the Hemolytic Anemia
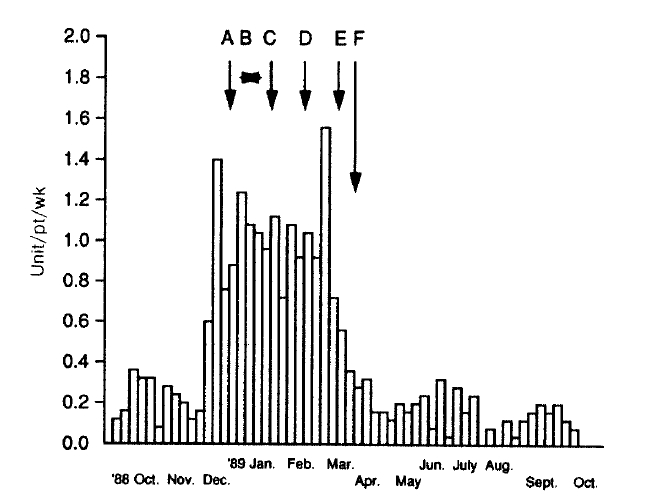
The water treatment system of the Hemodialysis Unit was composed of sediment filter, softner, charcoal column, reverse osmosis system and the storage tank. We changed the charcoal column, added ascorbic acid to the tank water (2 gm per 120 liter of water) for 2 weeks, changed the reverse osmosis system and the softener resin sequentially, but the episodes of hemolysis were not decreased at all (Fig. 3). We performed an analysis of the treated water that was obtained from the reverse osmosis system and the storage tank during hemolytic episodes. The results showed the concentrations of aluminum, copper, nitrate, sulfate and zinc within the standard levels for hemodialysis (Table 1). But the level of chloramine was 0.6 mg/L, distinctly exceeding the maximum permissible level of 0.1 mg/L16).
3. Analysis of Water from Different Water Sources
We further reviewed the ammonia nitrogen levels of our water filtration plant (A), which was located on the lower Han River and another water plant (B) located on the upper stream, to elucidate the cause of the high chloramine level. The ammonia nitrogen levels of water plant A were prominently higher than those of water plant B all around the year (Table 2). Compared with during the rainy season, the ammonia nitrogen levels were higher during the dry season. We measured the concentrations of residual chlorine before and after charcoal filtration from water plant A and B, respectively (Table 3). All the levels of total and combined chlorine in water from plant A were higher than those from plant B, regardless of charcoal filtration.
4. Period after Switching the Water Source
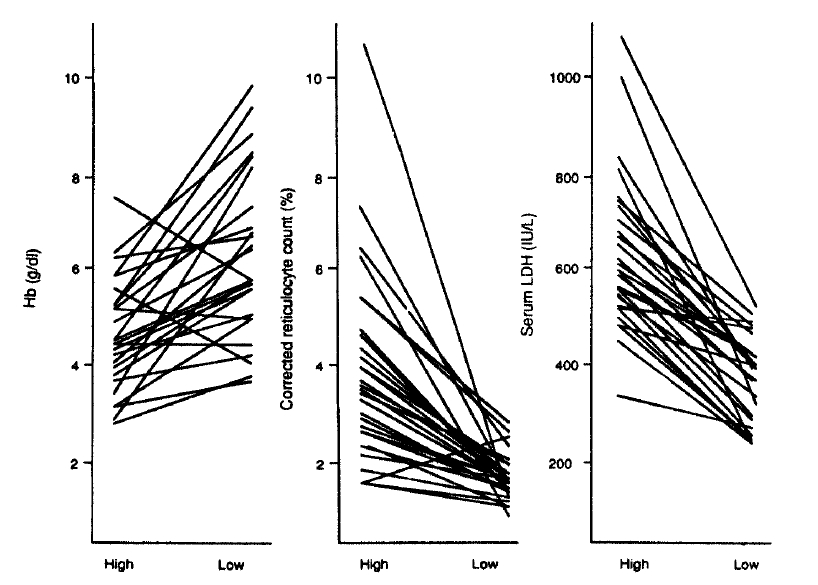
After switching the water source from A to B in April 1989, we re-evaluated the clinical and laboratory findings. The general conditions of the patients improved remarkably and the laboratory findings also showed distinct improvement. Hemoglobin levels of the patients increased from 4.8┬▒0.9g/dl during the hemolytic episode to 7.2┬▒1.4g/dl in May 1989. The levels of corrected reticulocyte and serum LDH were decreased from 3.4┬▒2.1% to 0.9┬▒0.5%, from 632.3┬▒156.4 IU/L to 381.3┬▒84.5 IU/L, respectively (Table 4 & Fig. 4). Plasma erythropoietin levels were decreased from 25.1┬▒1.1 U/L to 16.3┬▒6.2 U/L, and the rate of positive ascorbate cyanide test was decreased from 94% (30 of 34) to 15% (5 of 34).
DISCUSSION
Chloramines, which are oxidant compounds made up of chlorine and ammonia and widely used as bactericidal agents in urban water supplies, have been found responsible for the epidemics of acute hemolytic anemia characterized by Heinz bodies in hemodialysis patients10,11,13). Chloramines produce denaturation on hemoglobin (Heinz body), both by their direct oxidizing capacity and their ability to inhibit red cell reductive (hexose monophosphate shunt; HMPS) metabolism12). The HMPS, through generation of reduced nicotinamide adenine dinucleotide phosphate (NADPH), protects the red cell from oxidant damage. When this pathway is defective, red cells become vulnerable to damage from oxidant compounds. Pure chlorine in water can not cause these changes, but chloramine at the level of 0.25 mg/L invariably leads to hemolysis12).
From the beginning, chloramine-incuced hemolytic anemia was suggested by outbreak of hemolysis, methemoglobinemia and Heinz bodies on blood film in our patients. Diagnosis was made by high concentrations of chloramine in the treated water. Most of our patients showed positive Heinz body and ascorbate-cyanide test. Nitrates and copper can also induce hemolytic amemia7,8), but concentrations of nitrates and copper in the treated water in our center were within the standards for hemodialysis.
Chloramines represent a hazard in many dialysis centers since the reverse osmosis method of water purification does not remove these compounds. These compounds can be removed from water by adequate carbon filtration, but the disadvantage of expense and the danger of bacterial growth hide behind this method. Boiling and vacuum treatment are all effective but unacceptable in practice12). The control measures of chloramine-induced hemolysis in previous epidemics were by adding ascorbic acid to the bath (2g/120L of dialysate) in Minnesota12) and by fitting all dialysis machines with individual carbon filters in Sydney11). We reinforced the water treatment system by changing the charcoal column, setting up the carbon filter at individual dialysis machines and adding ascorbic acid to the tank water, but the episodes of hemolysis were not reduced. The unresponsiveness to the above mentioned procedures in our epidemic may be related to the enormous amount of chloramine in water. In fact, our bedside carbon filters could not effectively remove the residual chlorine in water. Recently, Cohen et al experienced the same situation as ours in S. Africa13). They also tried to neutralize the high chloramien in water with ascorbic acid and inserted charcoal filters ahead of the reverse osmosis system, but both methods were unsuccessful as in our case. They had to decrease chlorination of the municipal water to control the hemolysis.
Chloramines are preferred to chlorine in some western countries, as they have more stable chemical structure and do not evaporate as readily as chlorine. Chlorine is used as bactericidal agents in urban water supplies in Korea. However, residual chlorine can combine with ammonia in water, contaminated by raw sewage, and produce oxidant compounds, chloramine. Especially, in dry and winter seasons, the concentration of ammonia in water increases and the evaporation of residual chlorine decreases. Therefore, the hazard of chloramine increases in dry and winter seasons.
In conclusion, chloramine is one of the major contaminants causing dialysis-induced hemolytic anemia and regular determinations are necessary, especially during winter and dry seasons.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





