 |
 |
| Korean J Intern Med > Volume 6(2); 1991 > Article |
|
Abstract
Rupture of the heart as a complication of myocardial infarction is one of the most common causes of in-hospital mortality. Rupture of the free wall of the ventricle or interventricular septum has a poor prognosis when treated conservatively. So, rupture of the heart after infarction requires prompt diagnosis and early surgical repair despite the high overall incidence of early operative mortality before hemodynamic deterioration and multiorgan failures develop. Rupture of the left ventricle results in pseudoaneurysm if the overlying pericardium adhers to the surface of the heart. Pseudoaneurysms which rarely develop after infarction, tend to rupture. Their presence alone is an indicator for operation because of the very poor prognosis following rupture.
We experienced successful management of 2 rare complications after acute myocardial infarction: ventricular septal defect and pseudoaneurysm. The first patient was a 49-year-old man who had an apical septal defect. His electrocardiogram showed Q wave in leads V2ŌĆōV6, II, III, and aVF but a coronary angiogram showed normal findings. He was successfully treated by patch closure of the septal defect.
The second patient was a 65-year-old female who had false aneurysm of the left ventricle. She had neither chest pain nor abnormality on the electrocardiogram. A coronary angiogram showed complete occlusion of the distal circumflex artery. Under cardiopulmonary bypass, the neck of the aneurysmal sac was successfully closed with a prolene suture.
Key Words
Ventricular septal defect; Pseudoaneurysm; Acute myocardial infarctionRupture of the myocardium after acute myocardial infarction may involve the free wall of the ventricle, interventricular septum, or the papillary muscles. Rupture of the free wall usually results in either acute hemopericardium with apparent cardiac arrest or, rarely, when pericardial adhesions are present, in bleeding that is confined to a limited space, which gradually expands as the blood flows through a small communicating orifice under high pressure, forming a false aneurysm1). Besides the above types of ventricular rupture, ŌĆ£subacuteŌĆØ rupture may result in a syndrome resembling cardiogenic shock2). Septal rupture results in the creation of a left to right shunt.
As a result of the improved treatment of ventricular arrhythmia during the early phase of acute infarction, myocardial rupture has become, after myocardial power failure, one of the most common causes of in-hospital mortality3,4). Rupture of the heart as a complication of acute myocardial infarction is responsible for 5% to 13% of deaths after infarction5).
Postinfarction septal rupture has a poor prognosis when treated conservatively. Septal rupture after infarction should be considered for surgical repair unless a compelling reason exists to suggest otherwise6ŌĆō8). Pseudoaneurysm of the left ventricle in even asymptomatic patients or small-sized aneurysms, tend to rupture9). Their presence alone is an indicator for operation because of the very poor prognosis following rupture10).
This report describes the clinical features, diagnostic evaluation, operative management and pathologic findings of 2 patients with mechanical complications after myocardial infarction. One case is a patient with septal rupture after infarction. The other is a patient with pseudoaneurysm after infarction which followed pericarditis.
A 49-year-old man with a 3-year history of exertional chest pain was hospitalized due to acute myocardial infarction in June 1990. After an uneventful discharge, he still had exertional chest pain. In October 1990, he was admitted to another hospital due to progressive chest pain. Septal defect was found on echocardiography, but no specific management for septal rupture was done. After discharge, he had chest pain which was relieved by isonid spray. He was readmitted due to chest pain in December 1990 and referred to our hospital for definitive management of septal defect and postinfarction angina.
On physical examination, he was in relatively good condition and not dyspneic. Vital singns were blood pressure 100/70 mmHg, pulse rate 70 times per minute, and body temperature 36.2┬░C. The conjunctiva was not anemic. On chest auscultation, a pansystolic murmur was heard along the left sternal border, and the point of maximal intensity was ErbŌĆÖs area. On abdominal examination, the liver was palpated by 5 cm below the right costal margin.
At admission, a hemogram revealed hemoglobin 13.1 g/dl, WBC 5400/mm3, and platelet 142 k/mm3. Lipid profiles were cholesterol 149 mg/dl, triglyceride 114 mg/dl, and HDL 24 mg/dl. CK and LDH were within normal range.
A chest roentgenogram showed cardioimegaly and bilateral pleural effusions (Fig. 1). An electrocardiogram showed a Q wave in leads V3ŌĆōV6, II, III, and aVF, along with ST segment elevation in V3ŌĆōV5 (Fig. 2).
On echocardiogram, apical dilatation and apical septal defect through which a jet flow traversed were noticed (Fig. 3). Wall motions in the basal septum and left ventricular posterior wall were relatively well preserved and the ejection fraction was 80%.
Cardiac catheterization revealed increased main pulmonary arterial, right ventricular and right atrial pressures (MPA 64/18/32 mmHg, RV 60/2/18 mmHg, RA 18/18/14 mmHg). A left ventriculogram showed an apical aneurysm with apical and anterolateral wall hypokinesia and contrast filling to the right ventricle and pulmonary artery through interventricular septal defect. A coronary angiogram showed no definite abnormality. The pulmonary-systemic flow ratio was 3.76.
On the 9th hospital day, an operation was done. The patient was found to have pericardial effusion and septal defect which measured 1├Ś3 cm and were located in the apex. Fibrosis around the septal defect was noticed The septal defect was closed with a Dacron patch. The left ventriculotomy was closed with 2 Gore-Tex strips and buttressed by prolene horizontal matress suture. Finally, a Gore-Tex patch was attached to the LV wall. His postoperative recovery was uneventful, and echocardiography before his dicharge showed akinetic apical wall without septal defect.
On the 34th hospital day, he was discharged witout any complication and treated with digoxin, diltiazem, and isosorbide dinitrate at OPD.
On August 18 1990, a 65-year-old female was admitted to Seoul National University Hospital because of dyspnea, which devoloped 1 week before admission. She had been managed for diabetes mellitus for 20 years. On April 1990, she had been admitted because of pleuritic chest pain in her left anterior chest. At admission, pericardial friction rub was noticed on chest auscultation, and the electrocardiogram showed ST segment elevation in leads V1ŌĆō6, aVL, aVF, and II (Fig. 4). Her cardiac enzymes were within normal range. An echocardiogram showed concentric LVH with pericardial effusion. She was treated with antiinflammatory agents under the impression of uremic pericarditis and made a good recovery.
After discharge, her dyspnea progressed and pericardial effusion increased on echocardiogram which was done in OPD. So, she was readmitted due to progressive dyspnea and general weakness.
On physical examination, she was dyspneic, but showed an intact orientation and a clear consciousness, Vital signs were as follows: blood pressure 120/80 mmHg, pulse rate 72/min, respiration rate 26/min, and body temperature 36.3┬░C. The conjunctiva was pale and the jugular vein was markedly engorged. On palpation, the apical impulse was felt on the fifth intercostal space of the left anterior axillary line. On chest auscultation, the heart beats were regular without additional heart sound nor murmur. Inspiratory basal crackles were heard in her left lower lung field, especially on her posterior chest. Mild pitting edema in both extremites was noticed.
On admission a hemogram revealed hemoglobin 8.8 g/dl, WBC 10300/mm3, and platelet 212 k/mm3. Erythrocyte sedimentation rate was 41 mm per hour. Serum electrolytes were 128 mEq/l for sodium, 4.4 mEq/l for potassium, and 103 mEq/l for chloride. Arterial blood gas analysis revealed metabolic acidosis with compensatory respiratory alkalosis. Fasting blood glucose was 402 mg/dl. CK and LDH were within normal range. Blood urea nitrogen and cratinine were 35 mg/dl and 4.2 mg/dl, respectively.
A chest roentgenogram showed increased cardiomegaly and pleural effusion since the last study (Fig. 5).
An electrocardiogram showed no remarkable findings except nonspecific ST-T wave change (Fig. 6).
A 2-dimentional echocardiogram suggested a defect in the left ventrcular wall with a surrounding aneurysmal sac which contained a suspicious echogenic mass (Fig. 7). Lateral wall motion was decreased. The left ventricular dimensions were 55 mm in systole and 70 mm in diastole, and the ejection fraction was 51%.
Cardiac catherization showed elevated left ventricular end-diastolic pressure and pulmonary arterial pressure (LVEDP 30 mmHg, PAP; systole 60 mmHg, diastole 30 mmHg). A coronary angiogram showed complete occlusion of the distal circumflex artery, 30% stenotic lesion of mid-LAD, and 95% stenotic lesion of the posterior descending artery(Fig. 8).
The patient was transferred to the Intensive Care Unit after detection of pseudoaneurysm. On the 3rd hospital day, the initial pulmonary capillary wedge pressure was around 50/25 mmHg. Intravenous nitroglycerin and aminophylline along with furosemide were administered for control of congestive heart failure. Her blood pressure was maintained above 130/70 mmHg, but dyspnea continued with slight improvement.
On the 7th hospital day, an operation was done. The pericardial cavity contained effusion and loose adhesions. Severe fibrotic changes were found especially in the diaphragmatic surface which might suggest sequealae of tuberculous pericarditis. An aneurysmal sac the size of an adult fist was located in the posterolateral wall of the left ventricle. The neck of the aneurysmal sac measured 2.0├Ś1.5 cm, which was closed with prolene suture and reinforced by bovin pericardium.
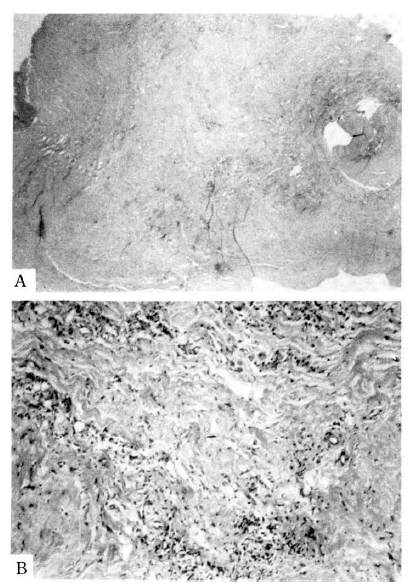
The aneurysmal specimen was found to be chronic nonspecific inflammation (fig. 9).
The pleural specimen was found to be chornic nonspecific inflammation with mild fibrosis.
On the 30th hospital day, she was discharged without postoperative problems. After discharge, she was managed through OPD for hypertension and mitral insufficiency which developed after the operation.
Ventricular septal rupture complicates 1% to 3% of all infarctions and reportedly accounts for 5% of all periinfarction deaths11). It occurs most frequently during the first week after myocardial infarction, the infartion often having been the patientŌĆÖs first attack12,13). Before operative repair was introduced by Cooley in 195614), septal rupture was almost always fatal. When medical therapy alone was used, 25% died from cardiogenic shock within 24 hrs and 80% died within 2 months12,13).
The onset of a new systolic murmur after myocardial infarction is the clinical sign that caused clinicians to suspect ventricular septal ruptue. In rare instances, a systolic murmur is absent or overlooked15). The diagnosis of ventricular septal rupture by means of bedside right-heart catheterization and sequential oximetry was described in 197216). This technique differentiated the septal rupture from infarction-induced acute mitral regurgitation, because septal rupture usually produces a pulmonary-to-systemic flow ratio of more than 2:117). If right-heart catheterization cannot be performed easily, septal defect sometimes can be visualized by 2-dimensional echocardiography with color flow image18). Left-to-right shunt also may be detected by radionuclide techniques19).
The approach to the management of patients with ventricular septal rupture has varied over the years. Until the late 1970s, most cardiologists and cardiovascular surgeons recommended waiting at least 3 to 6 weeks before attempting surgical repair of the septal rupture17). This was done primarily to allow myocardial healing and fibrosis to occur in the hope that this would facilitate repair. The overall incidence of early operative mortality is high20).
A delay in repair for 6 to 8 weeks results in an improved early mortality, but the cost is the death of a number of patients who might otherwise be salvaged by early operation. The current approach in most units, therefore, is to consider ventricular septal rupture as a surgical emergency6ŌĆō8).
Most studies postulated that perioperative survival is mainly determined by preoperative cardiogenic shock or right ventricular dysfunction21,22). Unlike most complications of myocardial infarction, the clinical outcome does not appear to depend on the left ventricular function, shunt size, infarct size or extent of the coronary disease21,22).
Several technical advances in the operation for ventricular septal rupture have been made in recent years. An anterior defect can usually be closed primarily with felt-buttressed sutures. Amputation of the apex and infarctectomy is often used if the defect is in the apical septum. Posterior septal defect usually requires prosthetic replacement of part of the posterior wall to prevent reopening of the defect or dehiscence of the ventriculotomy23ŌĆō25).
In our first case, a chest roentgenogram showed cardiomegaly and bilateral pleural effusion. An electrocardiogram showed a Q wave in the precordial leads and inferior limb leads, along with persistent ST-segment elevation in the precordial leads. We confirmed the presence of an apical septal rupture and apical aneurysm by echocardiogram and cardiac catheterization. The septal defect was closed with a Dacron patch, successfully.
The incidence of true aneurysm of the heart following myocardial infarction is approximately 10%26). The incidence of false aneurysm is not known but appears to be quite rare.
A true aneurysm develops by a gradual bulge of the involved portion of the wall. The process results from a removal of the infarcted tissue and concomitant molding of the bulge. The final result is an aneurysm with a wide mouth. The aneurysmal wall is comprised primarily of fibrous tissue among which elements of the original wall may be identified. Classically, the true aneurysm is relatively large, frequently 6 to 7 centimeters in diameter9).
A false or pseudoaneurysm is a consequence of the rupture of the ventricular wall but with containment of the resulting hematoma. With time, the periphery of the hematoma becomes organized into fibrous tissue among which no element of the cardiac wall is present. Characteristically, the mouth of a false aneurysm is narrow compared with the width of the fundus. In a false aneurysm, an important difference compared with a true aneurysm is in regard to the rupture of aneurysm27,28).
The clinical presentation of a patient with pseudoaneurysm may be pericarditis, cardiac failure or supraventricular tachyarrhythmia. The chest radiogram may show cardiomegaly with abnormal bulges from the ventricular wall. Echocardiography is of diagnostic value29). Angiography will determine whether an aneurysm is true or false and, in addition, show if there is appreciable coronary artery disease30).
The surgical management of false aneurysms is simpler than that of true aneurysms, as the excision of the left ventricular wall is not required because the aneurysm does not constitute a part of the functional left ventricular wall.
In our second case, Pericarditis occurred before myocardial infarction developed. So, when left ventricular rupture after myocardial infarction developed, the left ventricular rupture did not make cardiac temponade and resulted in pseudoaneurysm. Chest roentgenogram showed cardiomegaly and bilateral pleural effusions. Electrocardiogram and cardiac enzymes did not show typical findings for myocardial infarction. We confirmed a defect in the left ventricle wall with surrounding aneurysmal sac by echocardiogram. Before the operation, angiography was done for detection of appreciable mitral regurgitation that might require additional surgical treatment. The neck of aneurysmal sac was closed with prolene suture which was reinforced by bovine pericardium successfully.
Fig.┬Ā2
Electrocardiogram shows Q wave in leads V3ŌĆō6 and II, III, aVF, and ST segment elevation in V3ŌĆō5.

Fig.┬Ā3
Echocardiogram shows apical dilatation and apical septal defect through which a jet flow tranverses.

Fig.┬Ā7
Echocardiogram shows A) a rupture into the pseudoaneurysmal sac from the lateral wall of the left ventricle on apical 4-chamber view, and B) turbulent flow through the rupture site on apical 4-chamber view.

REFERENCES
1. Shabbo FP, Dymond DS, Rees GM, Hill IM. Surgical treatment of false aneurysm of the left ventricle after myocardial infarction. Thorax 38:25ŌĆō301983.



2. OŌĆÖRourke MF. Subacute heart rupture following myocardial infarction: clinical features of a correctible conditions. Lancet 2:124. 1973.


4. Fiedberg CK. Current status of the treatment of shock complicating acute myocardial infarction. J Trauma 9:141ŌĆō21969.

5. Friedman S, White PD. Rupure of the heart in myocardial infarction. Ann Intern Med 21:778ŌĆō821944.

6. Radford MJ, Johnson RA, Dagget WM, et al. Ventricular septal ruture: a review of clinical and physiologic features and an analysis of survival. Circulation 64:545ŌĆō521981.


7. Cohn LH. Surgical management of acute and chronic cardiac mechanical complications due to myocardial infarction. Am Heart J 102:1049ŌĆō601981.


8. Loisance DY, Cachera JP, Poulain H, Aubry P, Juvin AM, Galey JJ. Ventricular septal defect after myocardial infarction: early repair. J Thoac Cardiovasc Surg 80:61ŌĆō71980.

9. Vlodaver Z, Coe JI, Edwards JE. True and false left ventricular aneurysms, propensity for the latter to rupture. Circulation 51:567ŌĆō721975.


10. Stewart S, Huddle R, Stuard I, Schreiner BF, DeWeese JA. False aneurysm and pseudo-false aneurysm of the left ventricle. Ann Thorac Surg 31:259ŌĆō651981.


11. Lundberg S, Sodernstrom J. Perforation of an interventricular septum in myocardial infarction. Acta Med Scand 172:413. 1962.


12. Sanders RJ, Kern WH, Blount SG. Perforation of the interventricular septum complicating infarction. Am Heart J 51:736. 1956.


13. Wessler S, Zoll PM, Schesinger MJ. The pathogenesis of spontaneous cardiac rupture. Circulation 6:334. 1952.


14. Cooley DA, Belmonte BA, Zeis LB, Schnur S. Surgical repair of ruptured intervericular septum following acute myocardial infarction. Surgery 41:930ŌĆō71957.

15. Swithinbank JM. Perforation of the interventricular septum in myocardial infarction. Br heart J 21:562. 1959.



16. Meister SG, Helfant RH. Rapid bedside differentiation of ruptured interventricular septum from acute mitral insufficiency. N Engl J Med 287:1024. 1972.


17. Giuliani ER, Danielson GK, Pluth JR, Odyniec NA, Wallace RB. Postinfarction ventricular septal rupture. Circulation 49:445. 1974.

18. Harrison MR, MacPhail B, Gurley JC, Harlamert EA, Steinmetz , Smith MD, DeMaria AN. Usefulness of color doppler imaging to distinguish ventricular septal defect from acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol 64:697ŌĆō701989.


19. Gustafson A, Nordenfelt I, White T. Diagnosis of ventricular septal defect in acute myocardial infarction without cardiac catheterization. Acta Med Scand 198:471. 1975.


20. Keenan DJ, Monro JL, Ross KJ, Manners JM, Conway N, Johnson AM. Acquired ventricular septal defect. J Thorac Cardiovasc Surg 85:116ŌĆō91983.


21. Held AC, Cole PL, Lipton B, Goldberg RJ, Alpert JS. Rupture of the interventricular septum complicating acute myocardial infarction. Am Heart J 116:1330ŌĆō61988.


22. Moore CA, Nygaard TW, Keiser DL, Cooper AA, Gibson RS. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular fuction in determining survival. Circulation 74:45ŌĆō551986.


23. Dagget WM, Mundth EM, Gold HK, Leinbath RC, Buckley MJ, Austen WG. Early repair of ventricular septal defect complicating inferior myocardial infarction. Circulation 50:111ŌĆō1121974.
24. Dagget WM, Guyton RA, Mundth ED, Buckley MJ, McEnancy MT, Gold HK, Leinbach RC, Austen WG. Surgery for postmyocardial infarction septal defect. Ann Surg 186:260. 1977.



25. Dagget WM. Surgical management of ventricular septal defects complicating myocardial infarction. World J Surgery 2:753. 1978.


28. Ersek RA, Chesler E, Korns ME, Edwards JE. Spontaneous rupture of false left ventricular aneurysm following myocardial infarction. Am Heart J 77:677ŌĆō801980.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



