INTRODUCTION
It is generally accepted that acquired cystic kidney disease (ACKD) is a natural consequence of long standing end stage renal disease (ESRD) regardless of its underlying disease1). Recent reports about ACKD arouse clinicianŌĆÖs interest because it often lead to the complication of hemorrhage and it appear to increase the incidence of association with renal malignancy1ŌĆō3). However, the mechanism leading to cyst formation in ESRD remain uncertain3,4). Urokinase is a serine protease cleave the peptide bond Arg-560-Val-561 of plasminogen to produce plasmin which is the main component of the fibrinolysis system5,6).
It is also known that urokinase plays an important role in extravascular fibrinolysis such as tissue remodeling, cell migration7ŌĆō9) and degradation of the structural protein10,11). The urokinase production in the kidney is so great that the concentration in the urine is several times higher than in the plasma12ŌĆō14).
Recently, we reported15) that, as the renal mass decreases during the progression of ESRD, regardless of its underlying diseases, the remnant nephrons produce a larger amount of urokinase than the normal nephrons. With this result, if renal tubules were exposed to relatively high concentrations of urokinase, it might accelerate the cystogenic degenerative change of the renal tubules. This study was undertaken to see whether there is any difference in urine urokinase concentration between the ACKD group and non cyst (control) group in ESRD.
METHOD
1. Patients
Fiffy ESRD patients who had been maintained on chronic hemodialysis at Soonchunhyang university Chunan hospital for various periods of between 4 months and 70 months were chosen for this study. Causes for the underlying disease were various and ages ranged between 20 and 68 years old. The details of underlying diseases and sex distribution are summarized in Table 1.
2. Detection of Renal Cyst
Sonogram for the detection of renal cyst was performed by a radiologist (one of the authors of this study), using the 3.5 MHz linear and conex transducer (ALOKA SSD-270). Special attention was paid to rule out hydronephrosis and adult type polycystic kidney disease from ACKD.
3. Urokinase Activity
The urine urokinase activity was measured by a chromogenic peptide substrate, S-244416). 100 ul of urine, from 24 hours urine collection and standard urokinase in PBS were allowed to react with a defined amount of colorless substrate, S-2444 to release the colored p-nitroaniline which is measured spectrophotometrically at 405 nm. The absorbance was converted to urokinase activity (unit/ml) by the standard curve constructed from standard urokinase (product of Korean Green Cross CO.) in PBS.
4. Urokinase Concentration
It was derived from the following equation;
Our preliminary study showed that the urokinase activity was stable at room temperature for several weeks.
5. Statistics
Datas are expressed as mean┬▒one standard daviation of mean. Difference of urine urokinase concentration, dialysis duration and urine volume between groups were evaluated by the Mann-Whitney U test (between 2 groups), through the software stativew 512+ (Brain power, calabasas, CA) operating on a Macintosh PC. Statistical significance was considered to be present if p<0.05.
RESULTS
Out of fifty patients, nine patients have ACKD, five patients had polycystic renal disease (underlying disease of ESRD) and there was no cyst in thirty six patients (control group). The cyst (ACKD) was single in six cases, unilateral in eight cases and the diameter was less than 2 cm in ten cases (Table 2).
The duration of hemodialysis was 42.2┬▒17.0 month (range 19ŌĆō70 months) in the ACKD group, 39.0┬▒10.3 months (range 24ŌĆō54 months) in the polycyst group, and 20.0┬▒12.5 months (range 4ŌĆō53 months) in the control group. It was longer in ACKD group than the control group (p<0.005).
The 24 hour urine volume was 181┬▒131 ml (range 50ŌĆō400 ml) in the ACKD group, 450┬▒250 ml (range 100ŌĆō800 ml) in the polycyst group, and 697┬▒472 ml (range 100ŌĆō1,500 ml) in the control group. It was smaller in ACKD group than contro group (p<0.001) and polycyst group (p<0.05).
The urine osmolality was 323.3┬▒59.1 mOsm/L (range 250ŌĆō429 mOsm/L) in ACKD group, 311.2┬▒53.1 mOsm/L (range 237ŌĆō520 mOsm/L) in control group, 366.3┬▒70.6 mOsm/L (range 286ŌĆō419 mOsm/L) in polycyst group. There was no difference of urine osmolality between groups.
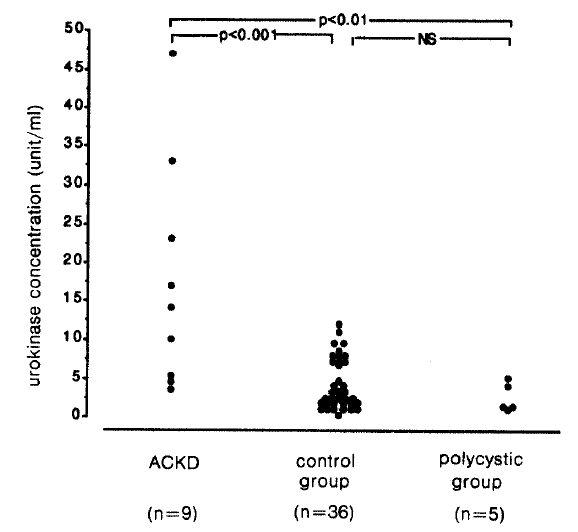
The urine urokinase concentration was 17.5┬▒14.7 unit/ml (range 3.5ŌĆō47 unit/ml) in the ACKD group, 2.6┬▒1.8 unit/ml (range 1.0ŌĆō5.1 unit/ml) in the polycyst group and 4.1┬▒3.4 unit/ml (range 0.5ŌĆō12.0 unit/ml) in the control group. It was higher in the ACKD group than the polycyst group (p<0.01) and control group (p<0.001), but there was no difference between the control group and polycyst group (Fig. 1).
The urine urokinase concentration showed a direct relation with hemodialysis duration and the longer the dialysis duration, the higher the urine urokinase concentration (r squared=0.424, p=0.0001) (Fig. 2).
DISCUSSION
Three hypotheses which exist for the mechanism of cyst formaiton in adult polycystic kidney disease17) may be applid to ACKD; 1) tubular obstuction due to epithelial hyperplasia with subsequent elevated transmural pressure leading to tubular dilatation; 2) increased tubular basement membrane compliance with tubular dilatation at normal transmural pressure leading to tubular dilatation; 3) increased radial growth of epithelial cells and basement membrane due to an unknown stimulus, resulting in dilatation in parts of the tubules.
There have been many reports supporting these hypothesis in ACKD18ŌĆō25), but non are coinclusive. Ishikawa et al26) reported two cases of ACKD which regressed after renal transplation; one recurred when the graft failed. This finding support a role for the uremic milieu or hemodialysis in the genesis of acquired cystic disease.
But there is good evidence that tubule obstruction could occur in ACKD, by epithelial hyperplasia19), intraluminal casts1), calcium oxalate deposits27) or tubular atrophy and associated interstitial fibrosis1). Whether tubule basement membrane compliance is increased in ACKD is unknown. If the increased compliance of basement membrane were the important factor for cystic degeneration in ACKD, possible factos suggested in literature, are hyperfiltration in remnant nephrons28), acute and chronic renal ischemia3), compensatory renal growth factor3), abnormal hormone level3), uremic toxin and some exogenous substance introduced by hemodialysis procedure18,20,29,30).
Urinary plasminogen activator is principally urokinase which is not filtered but is mainly produced in tubular epithelial cells31) and act on its main substrate plasminogen to produce plasmin. Considering that urokinase play an important role in tissue remodeling, cell migration7ŌĆō9), and structural protein destruction10,11) and ACKD arises from renal tubule of ESRD, the urokinase concentration in the tubule might be a cystogenic factor in some situation. The urokinase production was reported to decrease in ESRD32,33). Recently we found15) that as the GFR decreases, total urokinase in urine decreases, but the total urokinase divided by GFR (total u-PA/Ccr) increases abruptly when the GFR falls below 25L/day. This finding suggests that as the renal mass decrease, remnant nephron produce larger amount of urokinase than do normal nephron.
Even in ESRD, as long as urine formation is continued, there may be functioning nephrons on the way to functional loss. The deterioration of renal function will reach the point at which urine volume will be zero due to complete renal loss. We believe that this is the reasoin for decreased urine volume and longer duration of dialysis in ACKD group in our study. During this period, the tubules face a higher concentration of urokinase than the tubules of normal nephrons, and the longer the duration of ESRD (hemodialysis), the higher the expected urokinase concentration.
Our results show that the ACKD group had a higher concentration of urokinase and longer duration of hemodialysis than the control group.
With this concept, transformation of the control group into the ACKD group after some period of dialysis during which, remnant nephron produces much more urokinase is expected. Higher urokinase concentration in the ACKD group might be a result rather than a cause of ACKD. But there was no difference in the between the polycyst group and control group. This finding suggests that the increased urine urokinase concentration in the ESRD is rather the cause of ACKD than the result of ACKD.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





