INTRODUCTION
Leptospirosis is an uncommon disease that results from infection by one of the many serotypes of the spirochete Leptospira interrogans. In 1920, Takaki isolated Leptospira icterohemorrhagiae from a skunk for the first time in Korea1). Ro and others2) reported acute hemorrhagic lung disease of unknown causes in the fall of 1975, and since then there have been subsequent reports3,4). Although leptospirosis has long been thought to be present in Korea because of its occurrence in the neighboring countries of Japan, China and Taiwan5), it was not until 1984 that leptospirosis in humans was documented by serology6), by autopsy7), and by isolation of causative organism8).
Numerous combinations of symptoms and signs can occur. The degree of severity may vary from hepatorenal failure and meningitis associated with classic Weils disease to a mild flu-like illness. Leptospirosis is known to show protean clinical manifestations3,4). The authors here describe a sudden outbreak of leptospirosis in Chonbuk Province in the fall of 1987.
MATERIALS AND METHODS
1. Study Population
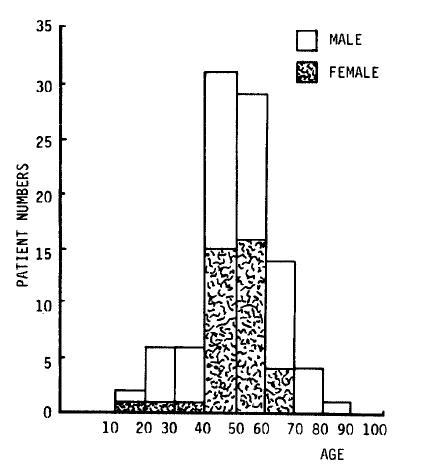
In this study, 93 patients were hospitalized at Chonbuk University Hospital, Chonju Presbyterian Medical Center, and Nam-Won Medical Center from September 10 to December 31, 1987. The age and sex distribution of the patients are shown in Fig. 1. All had acute febrile illness, the cause of which was suspected to the leptospirosis because of a similar outbreak after flood in 19859). In the same period in 1987, 31 patients of hemorrhagic fever with renal syndrome and 31 patients of scrub typhus were also recognized (Fig. 2).
2. Serologic Studies
Serum samples were screened by macroscopic slide agglutination test. All sera that agglutinated the screening antigens were differentially treated by microscopic agglutination test with 18 serotypes of leptospires. A titer of agglutination of >50% in a single specimen of a serum dilution of 1:80, or a four fold increase in titer in paired sera, were considered diagnostic of current infection. The serotype giving the highest titer was considered to be the infecting serotype.
The distribution of serotypes of leptospires identified by serum antibody response is shown in Table 1. Ten serotypes were found, but three serotypes of Leptospira icterohaemorrhagiae, canicola and CH-48 accounted for >90% of the cases (Table 1). Serovar lai was isolated in one patient at the National Institute of Health of Korea. Antibody responses of patients with leptospirosis measured by microagglutination test are shown in Table 2. Hemorrhagic fever with renal syndrome and scrub typhus were diagnosed by indirect immunofluorescent antibody test. Eight patients were both positive for leptospirosis and scrub typhus, and two were both positive for leptospirosis and hemorrhagic fever with renal syndrome.
3. Clinical Manifestations
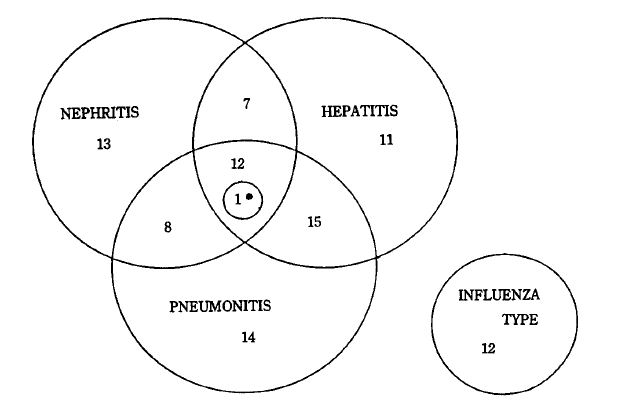
The onset of symptoms was severe and abrupt in most cases with fever, chill, headache, and myalgia. A fever was almost always present. A high fever of over 40┬░C was observed in 3% of the patients. Biphasic fever was noted in 18%. The average duration of fever was 6.8 days. The most prominent diagnostic symptoms and signs were leg pain on walking and muscle tenderness in the lower extremities. Blood-tinged sputum or hemoptysis was noted in 40% of the patients. Other manifestations are shown in Table 3. The patients were classified into four clinical types according to clinical, laboratory, and radiologic findings (Fig. 3). The pneumonitis type showed hemoptysis or chest lesions. The hepatitis type showed jaundice or abnormal live function test. The nephritis type showed abnormal urinalysis or increased BUN and creatinine. Patients who didnŌĆÖt belong to any type above were classified as influenza type.
RESULTS
1. Laboratory Findings
As shown in Table 4, 37% of the patients had moderate to severe anemia. The hematocrit and platelet count were lower in the group with hemoptysis than in the group without hemoptysis (Table 5). Leukocytosis and leukopenia were seen in 22% and 29%, respectively. Slight proteinuria was seen in 43%. The creatinine level was elevated in 15% of the cases. There was a marked elevation of ESR in the first few days in all cases. Blood chemistry showed mild hypoalbuminemia in 64% of the cases, increased level of bilirubin (usually direct bilirubin) in 21%, of SGOT in 50%, and of SGPT in 39%, and of lactic dehydrogenase and creatinine phosphokinase in 39% and 30%, respectively. Arterial blood gases analyses were performed in 35 cases. Mild metabolic acidosis and respiratory alkalosis were seen in 60% of the cases. Levels of PaO2 <55 mmHg and PaCO2<45 mmHg were seen in 31% of the cases.
2. Radiological Findings
Chest X-ray showed abnormal findings, including small patchy lesions, confluent areas of consolidation, and increased interstitial markings in 40 cases (43%). The chest lesions were divided into three categories according to their severity (Table 6). All of the five fatal cases showed severelly progressing forms on both lung fields. The chest lesions were unilateral in seven cases (18%) and bilateral in 28 cases (70%). The chest lesions showed right predominance in 25 cases (63%). Pleural effusion and cardiomegaly were seen in 12 cases (30%) and 13 cases (33%), respectively.
In the majority of the cases, resolution occurred from three to nine days after therapy with antibiotics. But in three cases, chest lesions showed aggravation before resolution. Resolution was almost always complete.
3. Necropsy Findings
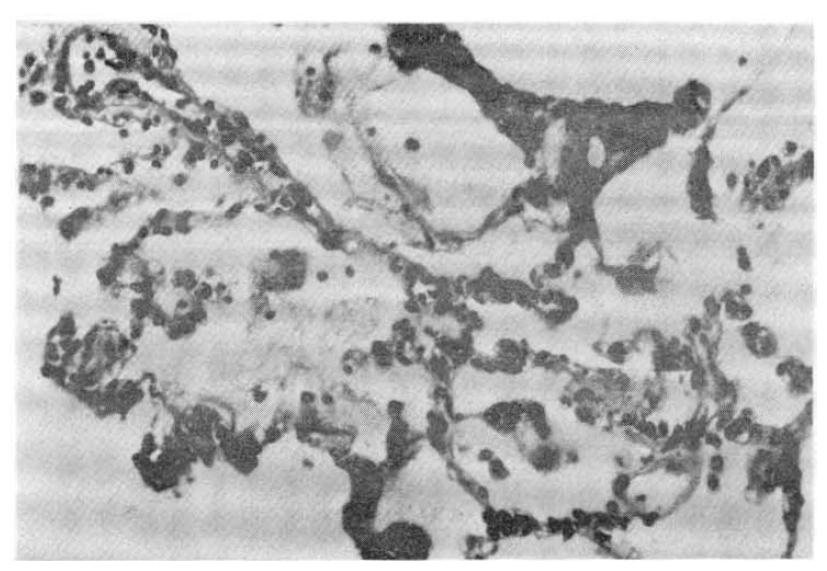
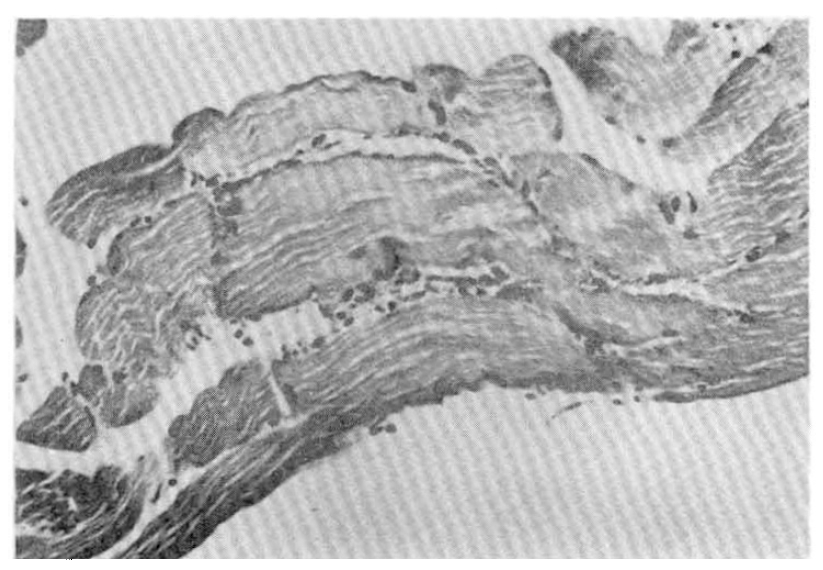
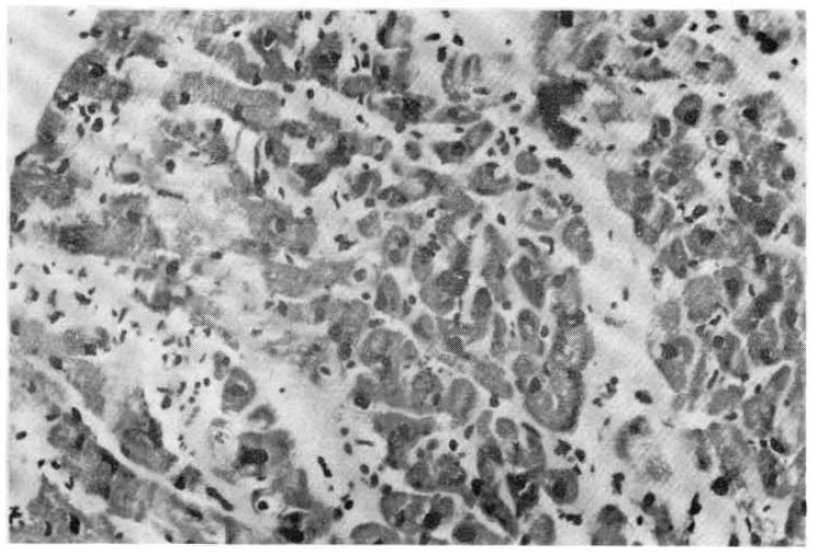
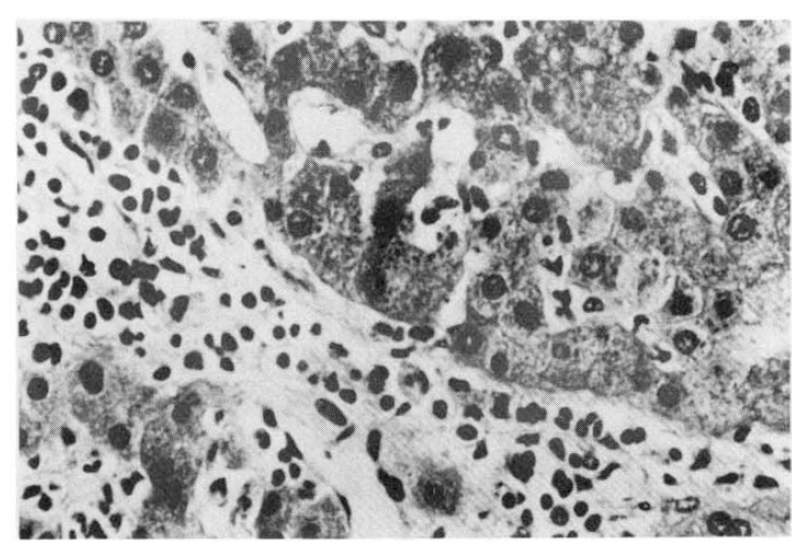
In the five fatal cases, the lungs showed septal congestion, multifocal alveolar hemorrhage, and fibrin exudation with a mild mononuclear cell infiltration (Fig. 4). The kidneys showed cloudy swelling and focal necrosis of proximal convoluted tubule and a mild interstitial edema (Fig. 5). The calf muscle showed interstitial edema and a focal collection of mononuclear cells with interstitial fresh hemorrhage and a myofiber degeneration (Fig. 6). The myocardium showed a mild interstitial edema, scattered large hyperchromatic nuclei suggestive of hypertrophic fibers and capillary congestion. Additionally, microscopic hemorrhage was noted in the interstitium together with focal hyalinization of the sarcoplasm. Perinuclear lipofuscin deposit was also seen. A few mononuclear inflammatory cells were seen around the dilated vessels (Fig. 7). The liver showed portal widening with some degree of fibrosis and inflammatory cell infiltration. The inflammatory exudates consisted mainly of lymphocytes, with a mixture of a few neutrophils and large mononuclear cells. Spotty necrosis of liver cells was also noted. Foci of microvascular changes of hepatocytes were seen (Fig. 8).
DISCUSSION
Leptospirosis is one of the most common zoonoses in Korea today. It occurs worldwide, and the true prevalence of leptospirosis has never been known accurately, since the disease is under-suspected and under-diagnosed.
Humans contract it by coming into direct contact with infected animal tissue or with soil or water contaminated by leptospira-laden urine10). The sudden outbreak of leptospirosis in September of 1987 seemed to be due to the wash-out of leptospira-laden rodents, urine into the fields where workers were tying up the fallen rice stalks after severe flooding4). The outbreaks of leptospirosis in 1975, 1984, and 1985 also occurred after severe floods before the harvesting season2,4). Table 1 shows the main three serogroups in the Chonbuk area. The most common was icterohaemorrhagiae in 1987, while in 1986 it was canicola. This suggests regional and yearly variations11,12). Definite diagnosis depends upon either the isolation of the pathogen or, more commonly, the specific serologic test. Because a culture system is usually not available, serologic test is an easy mean of diagnosis. However, as shown in Table 2, one should take into consideration the fact that serological responses in humans were sometimes slow in rising, and in cases in which antibiotics have been given early in the infection, a detectable immune response was delayed or blunted10,12,13).
All of the patients were farmers and their families who had worked in the fields more than once. Twenty percent of the patients sustained small cuts or abrasions on the hands and feet while working. These were ideal routes of leptospiral infection14). One case of simultaneous infection of husband and wife was seen. Various studies of leptospirosis have shown that infected males outnumbered females several times9,11,12,15,16). Male predominance has been thought to be due to the factors of occupation and environment11,16). As shown in Figure 1, this study showed the ratio of male to female to be 3:2. This increase in the female fraction was thought to be due to the increase of female workers in the fields, since many men were involved in damming up the flood or other repair work. As shown in Figure 2, there were overlapping periods of leptospirosis, hemorrhagic fever with renal syndrome, and scrub typhus. They needed careful differentiation because they were similar in early clinical manifestations, outbreak areas, time periods, and serologic tests.
Various combinations of symptoms and sings can occur during leptospira infection3,4,10,11,17,18). The most reliable clues for clinical diagnosis may be fever, muscle pain on stretching, and tenderness on palpation of the lower extremities, with or without hemoptysis. The authors clinically classified 54% of the patients as having the pneumonitis type, 49% as having the hepatitis type, 44% as having the nephritis type, and 13% as having the influenza type. The appearance of an influenza-like illness in the farmers of prevalent areas should alert practitioners of the possibility of leptospiral infection and the need to investigate these cases serologically. Our experience shows that leptospirosis in Korea is similar to that of China3,19) but differs clinically from that of other countries20,22). Overt meningitis was seen in only one patients. Abnormal liver function test was seen in 50% of the patients, 33% of which showed jaundice. No patient had renal failure severe enought to require dialysis. Hemoptysis was the predominant symptom. All fatal cases had chest lesions, and profuse, massive hemoptysis developed before death. The cause of death seemed to be asphyxia and acute respiratory failure. The mortality rate was 5% in this study, and was not related to age or to the severity of jaundice and renal failure, but to the severity of pulmonic lesion and massive hemoptysis. In three of the five fatal cases, the patients were transported over a long distance on unpaved roads. This may have been a causative factor, since in the prevention of hemoptysis absolute bed rest and avoidance of long-distance travel are important.
The pathogenesis of hemorrhagic pneumonitis is uncertain; since hemoptysis occurred in the early septicemic stage and a number of leptospira were found in the tissue of hemorrhahagic pneumonitis during autopsy, capillary damage by direct action of leptospiral toxins was suggested rather than disorders of the clotting mechanisms20,23,24). It is interesting that the extent of the lesions on the chest X-rays did not always coincide with the presence or absence of hemoptysis, or with the amount of blood expectorated23,24). Chest auscultation revealed less abnormal pulmonary findings than expected on radiologic studies9,24). Ten (27%) of the 37 patients with hemoptysis had negative chest X-rays, and 11 (25%) of the 40 patients whose chest X-ray showed lesions did not have hemoptysis. As shown in Table 5, patients with hemoptysis showed lower hematocrit and platelet counts than those without hemoptysis. The severity of anemia was roughtly proportional to the amount of hemoptysis. The causes of anemia were thought to be blood loss, microangiopathy and hemoptysis25). Thrombocytopenia, which was seen in 18% of the patients in this study, is a well-known complication of leptospirosis whose pathogenesis remains uncertain26). The authors have tried to find a relationship between thrombocytopenia and renal failure and jaundice, but failed to note any correlation.
As shown in Table 4, the erythrocyte sedimentation rate increased in all of the patients in the acute stage, remained high during the illness, and then decreased after convalescence. The degree of elevation appeared not to reflect the severity of the disease.
The determination of serum creatine phosphokinase may be a simple test to help diagnose the acute phase of leptospirosis27). In this study, it was elevated in 30% of the patients. Nonspecific electrocardiographic abnormalities were observed in 46% of patients with leptospirosis28,29), but the significance was not apparent.
Pulmonary function tests were performed in 25 cases. The abnormal findings included a mild obstructive pattern and a fixed obstructive pattern. All necropsies were performed just after death. The necropsy findings were similar to the previous reports in the Korean literature7,30).
Doxycycline and penicillin are both effective in shortening the course of illness when they are given in the first four days of illness31,32). Doxycycline, 100 mg once a week, will prevent leptospirosis in high-risk groups for three weeks33). Leptopiral vaccines have been available in Korea since 1988, but their effectiveness has not been determined. Control measures to eliminate the risks to farmers include vigorous rodent control, vigilant use of protective clothing, scrupulous personal hygiene, prophylactic use of doxycycline and possibly vaccines, and an awareness that leptospirosis is an occupational hazard, and that early diagnosis and prompt management can prevent a fatal outcome14,34).





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





