 |
 |
| Korean J Intern Med > Volume 2(1); 1987 > Article |
|
Abstract
To investigate the incidence of duodenal gastric metaplasia and its underlying gastric or duodenal diseases, the authors obtained endoscopic biopsy specimens from the duodenal bulb at random sites during endoscopy from 19 normal subjects, 11 patients with gastric ulcer, 18 with duodenal and/or prepyloric ulcer (s), 7 with duodenitis and 8 with gastric erosions. The biopsy specimens were assessed with PAS staining to confirm gastric metaplasia.
The incidence of duodenal gastric metaplasia was 72.2% in patients with duodenal and/or prepyloric ulcer (s), which contrasted with the patients with gastric ulcers (36.4%), duodenitis (42.9%), gastric erosions (12.5%), and normal subjects (5.3%).
In conclusion, the results suggest that gastric metaplasia seen predominantly in patients with duodenal ulcer, seems to be related to hyperacidity and it plays some role in the pathogenesis of peptic ulcer in duodenum.
Historically, presence of heterotopic gastric mucosa in the intestinal tract has never aroused particular interest among clinicians. Apart from its occurrence in the Meckel’s diverticulum, the condition is regarded as clinically insignificant. Gastric metaplasia in the duodenum has been described by several authors1–5), however, the significance of its occurrence remains unknown.
Extensive use of gastroduodenal fibroscopy enabled the authors to obtain biopsies of the duodenal mucosa. This paper will describe the incidence of gastric metaplasia in duodenum in various gastroduodenal diseases and discuss the pathogenetic role of gastric metaplasia in peptic ulcer.
The authors obtained two or three pieces of random biopsy specimens from apparently normal mucosa in the anterior wall of duodenal bulb using gastroduodenal fiberscope on 65 subjects. All biopsies were immediately fixed in 10% formalin solution, embedded in paraffin, and 5 mm thick sections were stained with haematoxylin and eosine and PAS, as described by Mark and Drysdale6) for sharp distinction of gastric metaplasia. The authors attempted to locate the fundic gland in all specimens but failed. Consequently, the possibility of gastric heterotopia was excluded.
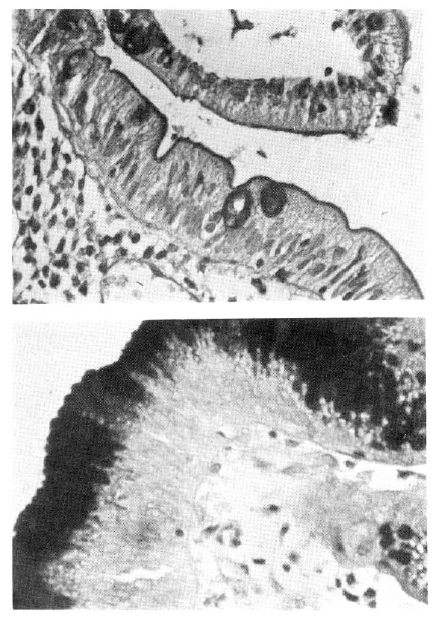
The identification of gastric metaplasia was relatively simple7). The surface epithelium of gastric mucosa stained strongly with PAS reaction contrasting sharply against the duodenal epithelium, where only goblet cells and the brush border disclosed a positive reaction (Fig. 1-a, 1-b).
The clinical characteristics of study cases are presented in Table 1. Duodenal ulcer and prepyloric ulcers are grouped together since they have the same pathogenetic mechanism as proposed by Johnson8).
The gastric ulcer group consisted solely of patients with ulcers located at the body, angle, or proximal antrum: two of the subjects in this group were additionally diagnosed with duodenitis.
The incidence of gastric metaplasia according to its underlying diseases is shown in Table 1. Gastric metaplasia was not observed in normal subjects with exception to one (5.2%). In patients with duodenal and/or prepyloric ulcer (s), its incidence was 72.2%, which was higher than in normal controls (5.2%) and those with gastric ulcer (36.4%), nevertheless the difference lacked statistical significance (p<.25).
Three of 7 patients with duodenitis (42.8%), and one of 8 patients with gastric erosions (12.5%), had gastric metaplasia.
The incidence of gastric metaplasia in the patients with gastic ulcer seemed to be varied according to the location of ulcer. Although the number of cases was too low to evaluate it more thoroughly, the incidence of metaplasia was higher (66.7%) in the patients with ulcers located in lower portion of the stomach (prepyloric and pyloric) than ulcers located in lower portion of the stomach (prepyloric and pyloric) than ulcers located in upper portion of the stomach (57.1% in those with ulcers located in angle and proximal antrum: 0% in those with body ulcer). The difference, however, was not statistically significant (p<.1).
The gastric mucosa in the duodenum can be classified into congenital heterotopic gastric mucosa and acquired gastric metaplasia as shown in Table 39). Until now, many authors have made the mistake of combining metaplasia and true heterotopic gastric mucosa10).
Since Taylor5) reported earlier (in 1927) two cases of gastric heterotopia in the duodenum, many authors described heterotopic gastric mucosa in duodenum as slightly raised patches consisting of chief cells and parietal cells. As true gastric heterotopia is frequently seen in Meckel’s diverticulum, and occasionally in rectum13) and small intestine, it is usually regarded as congenital in origin.
The gastric metaplasia, a term which was first suggested by Lessels10), is an incomplete form of gastric heterotopia, and is composed of foveolar and pyloric gland: it can be identified only with microscopy.
The incidence of true gastric heterotopia is suggested to be present in approximately 2% of the population10). However, microscopic gastric metaplasia is far more frequently seen than true heterotopia and it is especially common in duodenal ulcer patients. The incidence of gastric metaplasia in duodenal ulcer patients was reported to be 51.9% by Johansen4), 74.3% by Urakami11) and 72.2% by these authors series, which was quite similar to previous reports.
It is unknown whether the gastric metaplasia has a protective role or a harmful effect in the formation of duodenal ulcer. Johansen7) demonstrated a significant increase in frequency of lesion occurrence with the acid output. The presence of gastric epithelium in the duodenum could indicate a simple protective response to excessive acid secretion.
Urakami11) reported that the gastric metaplasia is less frequent in the active stage of duodenal ulcer, and its incidence increases in the ulcer’s healing stage. Jochi12) found that gastric metaplasia was frequently seen in the margin of the ulcer and appeared to occur with the healing process of the ulcer.
Suzuki3) divided the gastric metaplasia into three types: foveolar cell metaplasia, parietal cell metaplasia, fully developed fundic gland metaplasia: He reported that each incidence had been 79.2%, 10.4%. and 10.4% In these authors’ series, only foveolar gland metaplasia was found.
This study was intended only to reveal the incidence of gastric metaplasia in various gastroduodenal disease patients. The study could be expanded to clarify possible relations between acid secretion, stage of peptic ulcer, and stomach cancer.
Table 1.
Clinical Characteristics and Incidence of Gastric Metaplasia According to It’s Underlying Diseases
| Type of underlying diseases | No. of cases | Sex (M : F) | Age (Mean ± 1 S.D. yrs) | No. of cases with gastric metaplasia (%) |
|---|---|---|---|---|
| Normal | 19 | 9 : 10 | 42.2 ± 8.5 | 1 (5.2%) |
| G. U. * | 11 | 9 : 2 | 45.6 ± 10.8 | 4 (36.4%) |
| D. U. or prepyloric ulcer | 18 | 16 : 2 | 48.3 ± 11.0 | 13 (72.2%) |
| D. U. and G. U. | 2 | 0 : 2 | 53.0 ± 7.0 | 2 (100.0%) |
| Duodenitis | 7 | 5 : 2 | 43.8 ± 11.3 | 3 (42.8%) |
| Gastric erosion | 8 | 3 : 5 | 45.2 ± 5.6 | 1 (12.8%) |
|
|
||||
| Total | 65 | 42 : 23 | 45.0 ± 10.4 | 24 (36.9%) |
Table 2.
Incidence of Gastric Metaplasia in Gastric Ulcer*
| No. | No. with gastric metaplasia (%) | |
|---|---|---|
| Prepyloric and pyloric canal ulcer | 3 | 2 (66.7%) |
| Angle and proximal antral ulcer | 7 | 4** (57.1%) |
| Body ulcer | 4 | 0 (0 %) |
REFERENCES
2. James AH. Gastric epithelium in the duodenum of a patient with gastric hyperacidity. Proc 2nd Wld Conger Gastroenterology 2:540–5431963.

4. Johansen AA, Hart Hansen O. Macroscopically demonstrable heterotopic gastric mucosa in the duodenum. Scand J Gastroent 8:59–631973.


6. Marks TN, Drysdale KM. A modification of Zimmermann’s method for differential staining of gastric mucosa. Stain Technol 32:48–501957.


7. Johansen AA, Hart Hansen O. Heterotopic gastric epithelium in the duodenum and its correlation to gastric disease and acid? level. Acta Path Microbiol Scand Section A 81(5):676–6801973.

8. Johnson HD. Gastric ulcer: Classification, blood group, characteristics, secretion patterns and pathogenesis. Ann Surg 162:996–10041965.



9. Yoshimura H, Shida S. Heterotopic gastric mucosa in the duodenal bulb: A case report of father and son. Gastroent Endosc 27:750–7541985.
11. Urakami K. Gastric metaplasia in duodenal mucosa: Endoscopic and pathologic findings. Jap J Gastroent 72:221–2311975.
12. Jochi R, Maeda J, Yamashita K, Ichioka S, Tanoka S, Maruyama M, Takemoto D, Suzuki K, Kajwara I. A morphological study of gastric metaplasia in duodenal bulb. Progress of Digest Endosc 7:145–1481975.
-
METRICS

-
- 0 Crossref
- 2 Scopus
- 11,335 View
- 55 Download
- Related articles
-
The significance of ophthalmologic examination in a patient with xanthoma2024 March;39(2)
Increased risk of gastric cancer in workers with occupational dust exposure2021 March;36(Suppl 1)
The clinical usefulness of serum procalcitonin level in patients with scrub typhus2017 July;32(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


