 |
 |
| Korean J Intern Med > Volume 2(1); 1987 > Article |
|
Abstract
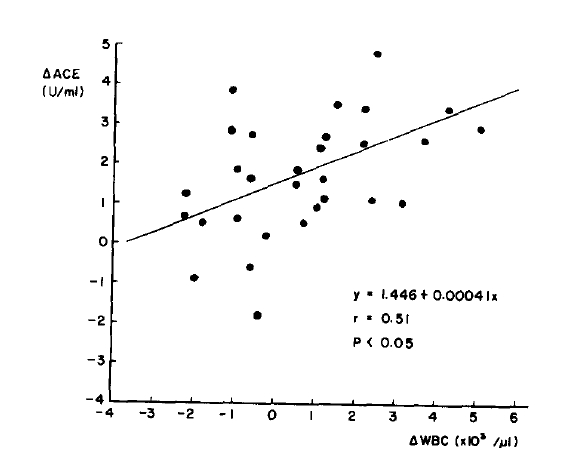
Plasma angiotensin-converting enzyme activity was measured by spectrophotometer in normal subjects and in patients with end stage renal failure, serially during a routine hemodialysis. Patients on maintenance hemodialysis tended to be associated with elevated plasma angiotensin-converting enzyme activity versus normal subjects. Plasma angiotensin-converting enzyme activity was significantly elevated in patients with chronic renal failure after 5 hours of hemodialysis(p<.001). Plasma angiotensin-converting enzyme activity corrected for hemoconcentration was also significantly increased(p<.05). There was a significant correlation between the increase in plasma angiotensin-converting enzyme activity after 5 hours of hemodialysis and the decrease in white blood cell count at one hour of hemodialysis (r = 0.51, p<.05). It is suggested that plasma angiotensin-converting enzyme analysis may prove to be a method for assessing transient pulmonary dysfunction during hemodialysis.
The activity of serum angiotensin-converting enzyme (peptidyldipeptidase; angiotensin IŌåÆII) has already been found to be elevated in the blood of patients with sarcoidosis1), GaucherŌĆÖs disease2), GravesŌĆÖ disease3), diabetes mellitus4), chronic renal failure5) and various types of liver disease6).
Angiotensin-converting enzyme(ACE) is normally abundant in endothelial cells especially in pulmonary vascular endothelium and renal tubules7). Acute damage to pulmonary vascular endothelium induced in animals by the administration of paraquat resulted in pulmonary edema and an increase of ACE in the blood which is thought to reflect the release of ACE from damaged endothelial cells8). During hemodialysis, pulmonary vascular damage may appear in the uremic patient, possibly caused by the plugging of pulmonary vessel by leukocytes9). The present study was undertaken to determine whether a single hemodialysis treatment causes an acute increase in the activity of ACE in the blood, thereby pointing to injury of the pulmonary vascular endothelium during hemodialysis.
The subjects in the study consisted of 24 healthy adult control(11 males and 13 females between 24 to 37 years of age; mean age 30 years) and 30 adults with end-stage renal failure on maintenance hemodialysis. The hemodialysis patients(16 males and 14 females) aged 27ŌĆō66 years(mean age 59 years) on chronic maintenance hemodialysis for durations of 7ŌĆō120 months(mean 35 months) were examined during a routine dialysis. The etiology of renal failure was as follows; chronic glomerulonephritis, 20 patients; diabetic nephropathy, 5 patients; polycystic kidney disease, 1 patient; renal tuberculosis, 1 patient; cervix cancer, 1 patient. The patients were dialyzed for 5 hours twice a week with hollow fiber capillary kidneys(GF 80H, 0.9 m2) using Gambro AK-10 dialyzers. Vascular access was provided by internal arteriovenous fistula and heparin was used for anticoagulation. The dialysate contained 134 mmol/l sodium and 33 mmol/l acetate. No patient was treated with an ACE inhibitor.
Blood samples for PO2, white blood cell (WBC) count, hematocrit and plasma ACE analysis were taken from the arterial line of the artificial kidney immediately before, at one hour, and after hemodialysis on all patients. Plasma samples were stored at ŌłÆ20┬░C for up to one week before assay. Plasma ACE was assayed by LiebermanŌĆÖs method1). This method employs hippuryl-L-histidyl-L-leucine as a substrate and determines the liberated hippuric acid by using a spectrophotometer.
Statistical significance was determined by the StudentŌĆÖs t-test. The correlation coefficients were determined by linear regression analysis. A p value less than 0.05 was considered significant.
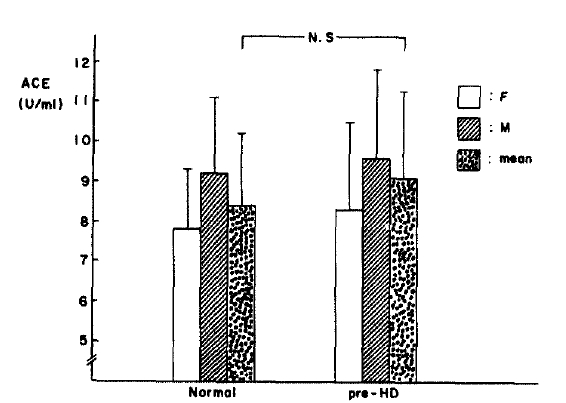
The changes in PO2, WBC count and hematocrit with dialysis time is shown in Table 1. The arterial oxygen tension fell from an initial value of 97.9 ┬▒ 11.6 mmHg to 95.5 ┬▒ 14.0 mmHg at one hour but increased to 100.6 ┬▒ 15.4 mmHg at the end of hemodialysis. Nevertheless it showed no statistically significant difference in PO2 change during hemodialysis. The WBC count also fell from initial value of 5,520/ul to 4,880/ul at one hour but significantly increased to 7,270/ul at the end of dialysis(p<.05) (Table 1). In normal subjects plasma ACE for males(9.2 ┬▒ 1.9 U/ml) showed higher values than females (7.8 ┬▒ 1.5 U/ml), and in patients with hemodialysis males (9.6 ┬▒ 2.3 U/ml) showed higher value than females(8.3 ┬▒ 2.0 U/ml). However, it did not show statistical significance respectively. Patients on maintenance hemodialysis tend to be associated with elevated plasma ACE activity versus normal subjects (Fig. 1).
Plasma ACE was significantly increased at the end of hemodialysis as compared to predialysis(p < .001). Plasma ACE was corrected for changes in hematocrit and showed a small but significant increase after 5 hours of hemodialysis(p<.05) (Table 2). The increase in plasma ACE activity at the end of hemodialysis did not significantly correlated to the PO2 drop at one hour after hemodialysis(Fig. 2). But there was a significant correlation between plasman ACE increment at the end of hemodialysis and drop in WBC counts at one hour after hemodialysis(r=0.51, p<.05) (Fig. 3).
It remains uncertain whether renal disease per se10) is associated with plasma ACE elevation or the hemodialysis procedure11) influences the enzyme level. One possibility is that the diseased kidney secretes excessive amounts of the enzyme in the blood. The is unlikely to be the case, however, since increased plasma ACE activity was also observed in a patients who had undergone bilateral nephrectomy5). A second possibility is that the enhanced ACE activity is a consequence of the failure of the kidney to excrete or degrade the enzyme. The high molecular weight of plasma ACE would argue against this possibility. Third possibility is that the increase in plasma ACE level in patients with hemodialysis may be due to diabetes mellitus4) or chronic liver disease6) in which an associated increased ACE level is known to exist. The fourth possibility is that hemodialysis procedure itself could increase plasma ACE level in patients with hemodialysis.
Craddock et al.9) showed the interaction between the blood and dialyzer membrane activates the complement system mainly by the alternative pathway leading to transient leukopenia during the first hour of dialysis therapy. An increase in complement fraction C5a was suggested to induce the formation of leukocyte aggregates and the embolization of these to the pulmonary vasculature causing pulmonary dysfunction with decreased diffusion capacity and increased small airway closing volume13).
Although localized in the vascular endothelium of several organs7), ACE is principally found in the lungs where it is concentrated on the luminal surface of the vascular endothelium in close contact with the blood and thus subject to pertubation by all factors that affect the integrity of this endothelium14). Our data showes that plasma ACE tends to be elevated in patients with maintenance hemodialysis. The further increase in plasma ACE following hemodialysis can be largely accounted for by the hemodialysis procedure.
The human ACE is a large, single polypeptide chain with an approximate molecular weight of 150,00015) and accordingly does not cross dialyzer membranes. In our study plasma ACE corrected for hemoconcentration also increased significantly after 5 hours of hemodialysis. We found an increase in plasma ACE during hemodialysis which was of the same order of magnitude and followed a similar time course as seen in animals with acutely induced damage to the vascular endothelium in the lung8). The increase in plasma ACE after 5 hours of hemodialysis is significantly correlated with the decrease in WBC counts at one hour of hemodialysis. But there was no significant correlation between plasma ACE changes and PO2 changes.
Dialysis associated hypoxemia is probably due to two concurrent process. In the earlier stages, the hypoxemia is related to the intrapulmonary leukostasis which is strongly dependent on the membrane material being used. This is superimposed on hypoventilation induced hypoxemia due to reduction in the CO2 load that occurs in acetate dialysis16). Since the decrease in PO2 during hemodialysis is not caused solely by leukocytes sequestration in pulmonary vessels, the increase in plasma ACE during hemodialysis more likely related to the change in WBC count than to PO2. Our study confirms that plasma ACE increases during hemodialysis are possibly due to vascular endothelial injury of the lungs.
Although further studies are needed to establish that the increase in plasma ACE during hemodialysis is selectively due to release of ACE from the lung, our result indicates that plasma ACE analysis may prove to be a new method for assessing pulmonary dysfunction during hemodialysis.
Fig.┬Ā2.
Relationship between the changes in ACE and the decrease in PO2 at 1 hour after hemodialysis.
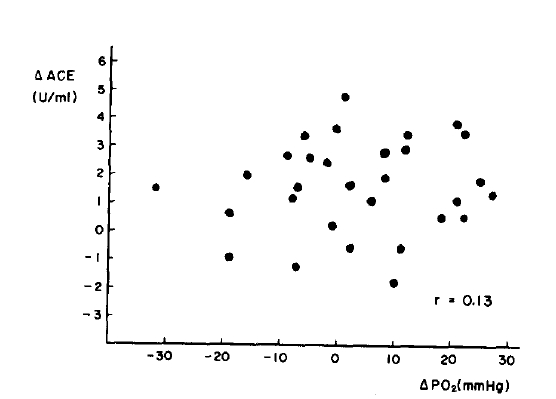
Fig.┬Ā3.
Relationship between the changes in ACE and the decrease in WBC count at 1 hour after hemodialysis.
Table┬Ā1.
Hematocrit, WBC Count and PO2, Changes During Hemodialysis
| Pre - HD | After 1 hr | Post - HD | |
|---|---|---|---|
| Ht (%) | 22.3 ┬▒ 4.9 | 20.6 ┬▒ 5.7 | 23.9 ┬▒ 6.6 |
| WBC (/ul) | 5,520 ┬▒ 1,310 | 4,880 ┬▒ 1,820 | 7,270 ┬▒ 2,610* |
| PO2, (mmHg) | 97.9 ┬▒ 11.6 | 95.5 ┬▒ 14.0 | 100.6 ┬▒ 15.4 |
REFERENCES
1. Lieberman J. Elevation of serum angiotensin-converting enzyme level in sarcoidosis. Am J Med 59:365. 1975.


2. Lieberman J, Beutler E. Elevation of serum angiotensin converting enzyme in GaucherŌĆÖs disease. N Engl J Med 294:1442. 1976.


3. Nakamura Y, Takerda T, Ishii M, Nishiyama K, Yamakada M, Hirata Y, Kimura K, Murao S. Elevation of serum angiotensin-converting enzyme activity in patients with hyperthyroidism. J Clin Endocrinol Metab 55:931. 1982.


4. Lieberman J, Sastre A. Serum angiotensin-converting enzyme; elevations in diabetes mellitus. Ann Intern Med 93:825. 1980.


5. Patel R, Ansari A. Serum angiotensin converting enzyme activity in patients with chronic renal failure on long term hemodialysis. Clinica Chimica Acta 92:491. 1979.

6. Schweisurth H, Wernze H. Changes of serum angiotension I converting enzyme in patients with viral hepatitis and liver cirrhosis. Acta Hepatogastroenterol 26:207. 1979.
7. Caldwell PRB, Seegal BC, Hsu KC, Das M, Soffer RL. Angiotensin-converting enzyme vascular endothelial localization. Science 191:1050. 1976.


8. Hollinger MA, Patwell SW, Zuckerman JE, Gorin AB, Parsons G, Giri SN. Effect of paraquat on serum angiotensin-converting enzyme. Am Rev Resp Dis 121:795. 1980.

9. Craddock PR, Fehr J, Brigham KL, Kronenberg RS, Jacob HS. Complement and leukocyte mediated pulmonary dysfunction in hemodialysis. N Engl J Med 296:769. 1977.


10. Silverstein E, Brunswick J, Rao TKS, Friedland J. Increased serum angiotensin-converting enzyme in chronic renal disease. Nephron 32:32. 1982.


11. Nielsen AH, Knudsen F, Kristensen SD. Serum angiotensin-converting enzyme increases during hemodialysis. Nephron 41:103. 1985.

12. Rumpf KW, Brat A, Amstrong V, Scheler F. Increased serum angiotensin-converting enzyme in end-stage renal disease. Nephron 40:248. 1985.


13. Craddock PR, Fehr J, Dalmasso AP, Brigham KL, Jacob HS. Hemodialysis leukopenia-pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest 59:879. 1977.



-
METRICS

- Related articles
-
Angiotensin converting enzyme inhibitors remain the first treatment of choice2016 March;31(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


