 |
 |
| Korean J Intern Med > Volume 12(2); 1997 > Article |
|
Abstract
We present a 30-year-old male patient who was initially diagnosed as minimal change nephrotic syndrome. 5 years later, the patient developed a localized form of idiopathic retroperitoneal fibrosis (IRF). An elevated ESR and concomitant nephrotic syndrome in the patient suggested the immunologic nature of IRF. IRF has been reported in association with collagen diseases and rarely with proliferative and nonproliferative glomerulopathies. To our knowledge, the association between minimal change lesion (MC) and IRF has not been reported. Furthermore, the fact that IRF presented itself as an abdominal mass and lacked systemic symptoms was also unusual.
Idiopathic retroperitoneal fibrosis (IRF) is a disease characterized by a fibrotic process at the retroperitioneal area and around the aorta. The fibrotic plaque entraps and gradually obstructs retroperitoneal structures such as the ureter, inferior vena cava and aorta.
IRF is believed to develop as a consequence of nonspecific inflammatory processes mediated by varying degrees of immune reaction. Associations between IRF and immune mediated diseases have been reported. About 15% of IRF patients have fibrotic process in areas other than retroperitoneal space. Elevated erythrocyte sedimentation rate (ESR), hypergammaglobulinema, autoimmune antibody, multiple vasculitis, pericardial or pleural effusion are frequently found in IRF.
Some links between IRF and renal disease, such as immune complex glomerulonephritis (GN), have also been reported. However, IRF and concomitant minimal change nephrotic syndrome is a rare finding. We present a young male patient with minimal change nephrotic syndrome who subsequently developed IRF 13 years later. Our IRF case is also unique in that the disease manifested as a localized abdominal mass with few systemic symptoms.
A 30-year-old man was admitted with a mass in the right upper quadrant (RUQ) of the abdomen. 13 years ago, he had generalized edema and ascites. Nephrotic syndrome was diagnosed and prednisolone and diuretics were prescribed. Even though his initial nephrotic syndrome had reached a complete remission, he had to take steroids intermittently because edema usually relapsed with steroid tapering. Five years ago, percutaneous renal biopsy was performed. Under light microscopy, his renal tissue showed no abnormal findings except slightly increased size in all 15 glomeruli (Fig. 1). Trace amount of mesangial IgM deposits were found on immunofluorescent microscopic Examination and diffuse effacement of foot processes were noted by electron microscopy. Under the impression of minimal change disease, the doctor advised the patient to take steroids as before.
Eighteen months ago, he felt a mass in the RUQ. The mass was not painful, but caused mild RUQ discomfort while growing in size. He was free from febrile sense, chill or other systemic symptoms. He denied any past history of abdominal trauma or surgery of having taken any medications such as herb, ergot derivatives, antihypertensives etc.
On admission, he was a slightly obses man weighing 71kg. His blood pressure was 110/70 mmHg, respiration rate was 20/min, and the body temperature was 36.5┬░C. A hard, fixed, palm-sized mass was palpated at the RUQ of the abdomen. Edema of a moderate degree was observed at both lower extemities. Other physical findings were not significant.
Laboratory data at the time of admission were listed in Table 1. He had massive proteinuria (19.8g/day) and a low serum albumin concentration (1.8g/dl). His serum cholesterol level was elevated to 382mg/dl. An elevated erythrocyte sedimentation rate (95mm/hr) and C-reactive proteins (2+) were also noted.
Computed tomography (CT) of the abdomen showed a huge 10cm-sized solid mass in front of and adjacent to the right kidney. The lobulated mass pushed the right kindey upward. Precontrast CT scan showed that the mass had heterogenous attenuation and fine calcification in it. The post-contrast scan revealed that the mass was a mixture of solid portions with high attenuation and necrotic portions with low attenuation. The outer margin of the mass was discrete. No intra-abdominal lymph node enlargement was seen (Fig. 2). On intravenous urography, the right kidney was rotated upward on the frontal plane but the shape and the excretory function were normal (Fig. 3). He was subjected to an exploratory laparotomy for the definitive diagnosis of intra-abdominal mass.
During laparotomy, we observed a well-encapsulated whitish solid mass with the dimensions of 15├Ś11├Ś19cm below the right kidney in the retroperitoneal space. The right kidney was pushed upward and rotated horizontally by the mass. In some part of the mass, the capsule was adhered to the posterior portion of the right kidney. The resected mass weighted 630g and its surface was tabulated. Cross scetions showed cystic and mucoid degeneration in the fibrotic bundles (Fig. 4).
No evidence of intra-abdominal lymph node enlargement or other organ abnormality was observed.
Seen through the light micorscope, most part of the tissue had dense deposits of collagen fiber and fibroblast proliferation but, at the periphery of the mass, there were localized inflations of inflammatory cells such as lymphocytes, plasma cells and histiocytes, forming germinal centers (Fig. 5). These findings were consistent with ŌĆśidiopathic retroperitoneal fibrosis (OrmondŌĆÖs disease).
After surgery, he was given 60mg of prednisolone q.d. for eight weeks. He reached remission at the end of the 8th week, but the nephrotic syndrome relapsed one month after prednisolone tapered off. Cyclophosphamide was added and currently he is maintaining a complete remission status for 20 months.
Retroperitoneal fibrosis is considered idiopathic in 70% of the cases, and the remaining 30% are attributed to various cause-medications (ergot derivatives, beta-blockers, methyl dopa), malignancy, retroperitoneal infection, trauma and abdominal aortic aneurysm1).
It has been suggested that IRF is an immune mediated disorder. Many IRF cases have been reported in relationships to various immunologic phenomena. Que et al reported a case of IRF with RaynaudŌĆÖs phenomenon, pulmonary fibrosis and anti-nuclear antibody in the serum and implied that IRF is a local manifestation of systemic fibrosing processes2). Lipman reported the case of a 29-year-old Negro woman with nephrotic syndrome and other characteristic features of systemic lupus erythematosus who developed IRF five years later3).
Kay described a case with retroperitoneal fibrosis and symptoms of arthralgia, stiffness and pericardial friction rub and changes of rheumatoid spondylitis at autopsy4). Although not evidently seen in this presented patient, about 15% of IRF cases are accompanied by fibrotic process in areas other than retroperitoneal space-i.e. mediastinal fibrosis, RiedelŌĆÖs fibrosing thyroiditis, sclerosing cholangitis, fibrotic orbital pseudotumors. These fibrosing lesions share definite pathological similarities with one another. Elevated erythrocyte sedimentation rate (ESR), hyperglobulinemia, autoimmune antibody (anti-thyroid antibody, anti-nuclear antibody, positive CoombŌĆÖs test), multiple vasculitis, pericardial or pleural effusion, immune-complex mediated glomerulonephritis5,6) are frequently seen in IRF, suggesting that IRF is a kind of connective tissue disorder and is a local manifestation of systemic diseases of an immunologic etilolgy3,6,7).
Association of IRF and renal disorder has been reported by a few authors. In a retrospective study8), among 46 IRF patients 13 patients (28.2%) had albuminuria. Two IRF cases with clinically evident nephrotic syndrome were also reported by Lipman3) and Liftman9). Renal biopsy findings of LipmanŌĆÖs patient were compatible with lupus nephritis in which focal proliferative lesions were intermingled with zones of fibrinoid necrosis3). LittmanŌĆÖs patient developed nephrotic syndrome 9 years after he was diagnosed to have IRF, and later his disease proved to be a renal amyloidosis9). Katz et al5) reported a 26-year-old female IRF patient whose renal tissue showed capillary and mesangial deposits of IgG and complements, which advocated a possibility of immune-complex mediated chronic glomerulonephritis in IRF. Electron dense deposits in the glomerular basement membranes observed by Zabetakis in a patients with crescentic GN and IRF also supports the possibility of immune-complex mecdiated GN in IRF6).
To our knowledge, no reports have been found about the association of minimal change nephrotic syndrome with IRF. Minimal change lesion is currently considered as a T cell mediated disease. The presence of CD4+ lymphocytes in IRF10, 11) and clinical efficacy of corticosteroids12ŌĆō15) when given before the dense fibrotic stage, as well as a case reported by Kirschbaum et al7), who had IRF and interstitial nephritis, support the possible role of cell mediated immunity in the pathogenesis of IRF. Therefore, it remains to be solved whether, as seen in our patient, minimal change nephrotic syndrome is pathophysiologically related with or a casual finding of IRF.
The second unique finding in this patient was that the presented patient had few systemic manifestations. Patients with retroperitoneal fibrosis usually have nonspecific symptoms-noncolicky back or abdominal pain, fatigue, weight loss and mild fever1,8). The symptoms and signs of the disease are closely associated with entrapment and mechanical compression of the ureter, inferior vena cava, aorta and its branches and gonadal arteries. The ureter is the most commonly involved structure. Ureteric obstruction by extrinsic compression progresses slowly and sometimes offers no symptoms. But, it casues flank pain and tenderness, costovertebral angle tenderness, oliguria or anuria and finally renal failure. Compression of the inferior vena cava can cause edema in both legs, the scrotum, and deep vein thrombophlebitis of the lower extremities. Eventually, compression of the aorta and common iliac arteries brings claudication and gangrenous change1,8). Our patient did not have any of the symptoms mentioned above, partly becasue IRF was localized to the RUQ with minimal compressive effects on the retroperitoneal structures, and partly becasue the steroids that he had been taking masked systemic symptoms of IRF.
IRF sometimes occurs in areas where there is a severe atherosclerotic change and weakened media in the aortic wall. This is explained by the hypothesis that insoluble lipid (ceroid) leaks from the aorta through the weakened wall and is phagocytized by macrophages, thus causing various immune responses and fibrosis in the end. But this hypothesis cannot account for the IRF in the children who do not have any atherosclerotic change in the aorta. IRF usually manifests as diffuse fibrosis around the descending aorta. IRF presented as a local mass, as in our case, is extremly rare. Chi reported, in 1978, a 42-year-old male patient with IRF16). His chief complaint for one year was a palpable nontender mass in the left upper quadrant of the abdomen. He was subjected to exploratory laparotomy for the differential diagnosis of the retroperitoneal mass. The mass was localized and surrounded the left kidney, 25├Ś17cm and well-demarcated. Other reports on localized forms of IRF cases were given by Judith17) and Thomas G18) (Table 2). Such localized forms of IRF should be differentiated from renal cell carcinoma, renal cortical or medullary carcinoma, lymphosarcoma, ureter tumors, pancreatic cancer, rectal cancer and intrapelvic tumor or abscess.
Ureterolysis is the treatment of choice for IRF. Ureterolysis involves dissecting the ureter from surrounding fibrotic plaque and wrapping it with peritoneum or omentum (intraperitonealization) in order to prevent it from re-fibrosis. Steroids are effective only at the early stage of the disease containing cellular infiltrates but does not help much in advanced fibrotic phases with scanty infiltrating cells. (Early stages are suggested by the findings of an elevated ESR, eosinophilia, and lymphocytic rather than fibroblastic infiltration in the biopsied plaque.) Azathioprine is another choice when steroid therapy is ineffective. Some authors reported that comcomitant long-term treatment with steroid and azathioprine induced loss of retroperitoneal mass and finally remission of hydonephrosis and lower leg edema19). Alternative therapies involve uses of indwelling urinary stent, percutaneous nephrostomy and percutaneous balloon dilatation.
Notes
This report was supported by a grant (No. 01-94-35) from the Seoul National University Hospital Research Fund
Fig.┬Ā1.
Light microscopy shows a slight increase in size of the glomeruli but otherwise no abnormal findings are observed (H&E ├Ś100).
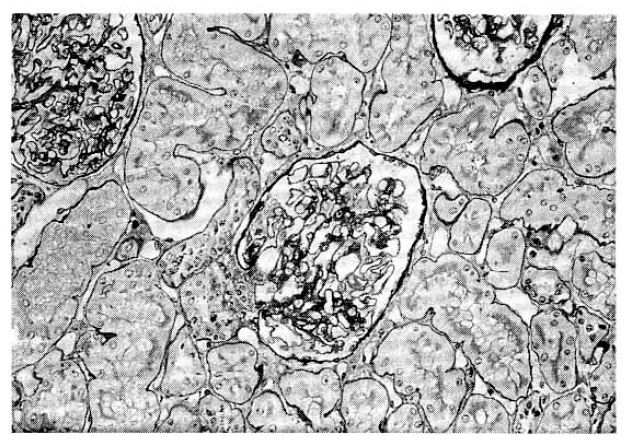
Fig.┬Ā2.
Postcontrast CT scan reveals that the mass is a mixture of highly attenuated solid portions and low-attenuated necrotic portions. The outer margin of the mass is discrete and no intra-abdominal lymph node enlargement is observed.
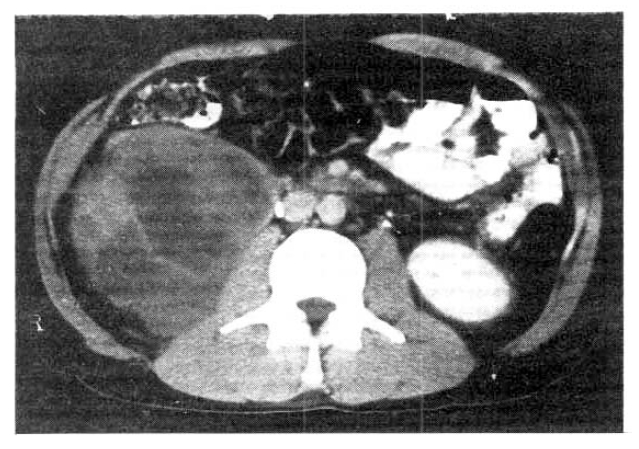
Fig.┬Ā3.
Anintravenous urography, the right kidney is rotated upward on the frontal plane, but no abnormal finding is seen in the shape of calyx, pelvis and ureter, nor the excretory function of both kidneys.
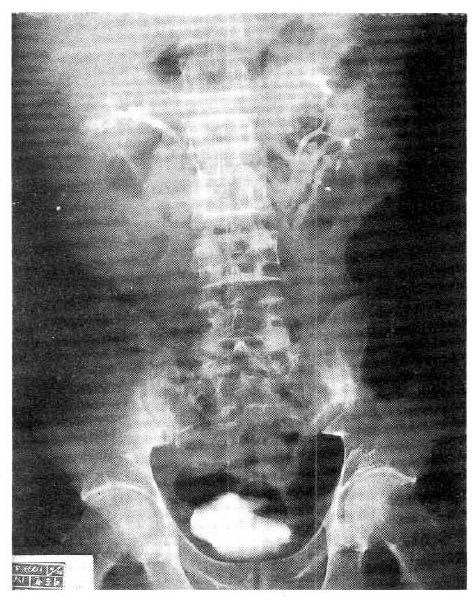
Fig.┬Ā4.
A cross surface of the removed mass shows it to be a fairly well-circumscribed mass with fascicles of fibrous tissue and two areas of cystic change.
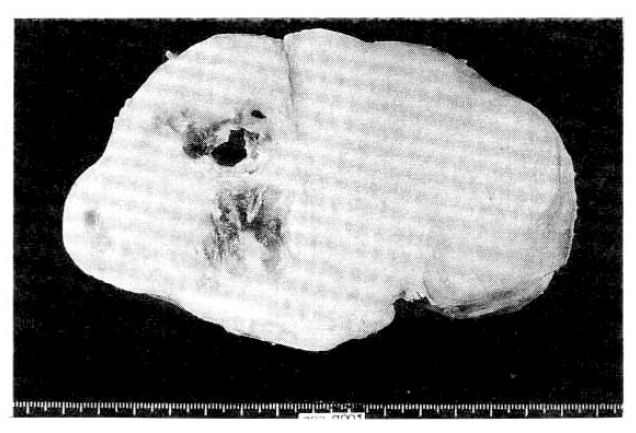
Fig.┬Ā5.
Photomicrographs of the mass show irregular fibrous bands and scattered inflammatory cell collections (H&H ├Ś100).
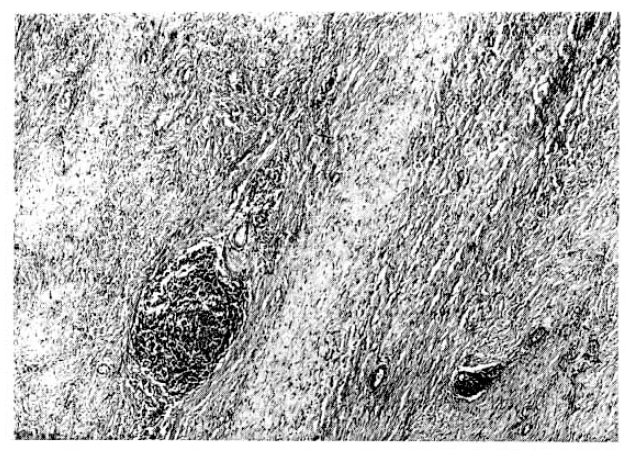
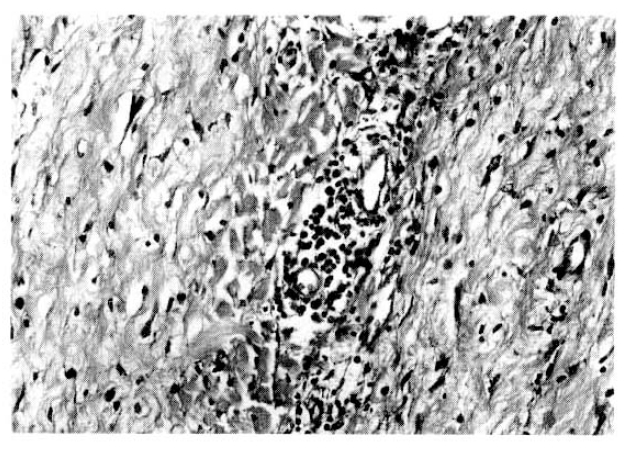
Fig.┬Ā6.
At a higher magnification of the fibrous mass, the inflammatory cells consist of lymphocytes and some plasma cells (H&E ├Ś350).
Table┬Ā1.
Laboratory Data of the Patient at the Time of Diagnosis of IRF
Abbreviations: CBC, common bood count; WBC, white blood cell; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; RBC, red blood cell; HPF, high power field; HBsAg, hepatitis B surface antigen; anti-HBsAb, anti-hepatitis B surface antibody; HCV, anti-hepatitis C virus antibody; VDRL; venereal disease research laboratory
REFERENCES
2. GS Que, Mandema E. A case of idiopathic retro-peritoneal fibrosis presenting as a systemic collagen disease. Am J Med 1964;36:320ŌĆō329.


3. Richard L, Lipman B, Johnson G, Berg A, Shapiro P. Idiopathic retroperitoneal fibrosis and probable systemic lupus erythematosus. JAMA 1966;196:204ŌĆō206.

5. Katz SM, Bates O, Yudis M, Falkner B, Griffith E. Immune complex glomerulonephritis in a case of retroperitoneal fibrosis. Am J Clin Pathol 1977;67:436ŌĆō439.


6. Zabetakis Paul M, Novich RK, Matarese RA, Michelis MF. Idiopathic retroperitoneal fibrosis: A systemic connective tissue disease? J Urol 1979;122:100ŌĆō102.


7. Kirschbaum Barry B, Koontz WW, Olicheny MJ. Association of retroperitoneal fibrosis and interstitial nephritis. Arch Intern Med 1981;141:1361ŌĆō1363.


8. Baker LRI, Mallinson WJW, Gregory MC, Menzies EAD, Cattell WR, Whitfield HN, Hendry WF, Wichham JEA, Joekes AM. Idiopathic retroperitoneal fibrosis. A retrospective analysis of 60 cases. Br J Urol 1988;60:497ŌĆō503.

9. Littman Edward. Renal amyloidosis with nephrotic syndrome associated with retroperitoneal fibrosis. Ann Intern Med 1971;74:240ŌĆō241.


10. Hughes D, Buckley PJ. Idiopathic retroperitoneal fibrosis is a macrophage-rich procss. Implications for its pathogenesis and treatment. Am J Surg Pathol 1993;17:482ŌĆō490.


11. Parums DV, Dunn DC, Dixon AK, Michinson MJ. Characterization of inflammatory cells in a patient with chronic periaortitis. Am J Cardiovasc Pathol 1990;3:121ŌĆō129.

12. Ochsner MG, Brannan W, Pond HS, Goodlet JS Jr. Medical therapy in idiopathic retroperitoneal fibrosis. J Urol 1975;114:700ŌĆō704.


13. OŌĆÖRegan R, Treahy PA, Prior IA. Idiopathic retro-peritoneal fibrosis. A case presenting with renal failure, treated effectively with adrenal steroids. N Z Med J 1961;60:518.

14. Morandi LP, Grab PJ. Retroperitoneal fibrosis: Response to corticosteroid therapy. Ann intern Med 1971;128:295.

15. Michinson MJ, Withycombe JF, Jones RA. The response of idiopathic retroperitoneal fibrosis to corticosteroids. Br J Urol 1971;43:444ŌĆō449.

16. Chi JeG, Chang JaJ. A case of idiopathic retro-peritoneal fibrosis involving the left kidney. The Seoul Journal of Medicine 1978;19:96ŌĆō100.
17. Yancey Judith M, Kaude Juri V. Diagnosis of perirenal fibrosis by MR imaging. J Comput Assist Tomogr 1988;12:335ŌĆō337.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



