 |
 |
| Korean J Intern Med > Volume 12(2); 1997 > Article |
|
Abstract
Objectives
To determine the effectiveness and toxicity when levamisole was added to the adjuvant combination chemotherapy in patients with operable gastric cancer.
Methods
After en bloc resection of gastric cancer without gross or microscopic evidence of residual disease from April 1991 to December 1992, 100 patients were randomized to 6 months of 5-fluorouracil 1,000mg/m2/day administered as continous infusion for 5 days, cisplatin 60mg/m2/day as intravenous infusion for 1 day with or without levamisole (50mg every eight hours P.O for a period of three days every 2 weeks for 6 months). This chemotherapy treatment was begun within 2 to 4 weeks after the surgery. The chemotherapy consisted of discrete 5-day courses administered at 4-weeks intervals. All 100 patients are assessable.
Results
The fifty patients were assigned to each treatment group. There was no statistical difference and no bias in the distribution of characteristics of the 100 evaluable patients between the two groups. A total of 274 courses of treatment were given in the levamisole group and 260 courses of treatment in non-levamisole group. Eleven patients in each group did not finish planned 6 courses of treatment mainly due to non-compliance. At median follow up of 39 months, 32 patients relapsed 19 in the levamisole group and 13 in the non-levamisole group (p=0.284). Twenty five patients died of relapsed diseases, 15 in the levamisole group and 10 in the non-levamisole group. The levamisole group tended to show more risk of overall death rate and recurrence than the non-levamisole group. Howerer. this result was not statistically significant at 3 years. The treatment was well tolerated in both treatment groups. The grade 2ŌĆō3 toxicites were nausea/vomiting (levamisole, non-levamisole group;31.7%, 29.3% of treatment courses respectively), diarrhea (7.6%, 8.4%), mucositis (11.6%, 12.3%), and leukopenia (9.8%, 9.6%).
Conclusion
Levamisole had negative effects on disease-free survival and overall survival when added to adjuvant combination chemotherapy of cisplatin and 5-fluorouracil in patients with operable gastric cancer. Both treatment arms were generally well tolerated and the toxicity profile was similar with or without levamisole.
Although surgeical treatment is the most effective treatment for gastiric cancer, the adjuvant chemotherapy is of prime importance for the prevention of postoperative recurrence and the prolongation of survival.
Advances in surgical techniques improved the therapeutic results for gastric cancer, yet it reached its limit for the patients who had advanced disease. Radiotherapy has no effect against gastric cancer. Chemotherapy is not yet satisfactory as a treatment for advanced gastric cancer.
Reccently Immunotherapy has shown promised as another therapeutic modality by improving the survival of the patients with gastric cancer.
Levamisole was originally developed as an anthelmintic. Since Renoux et al1). reported increasd immune response of the host after receving levamisole, Levamisole has been known to have a broad range of immunmodulatory effects. It includs the enhancement of antibody production to several different antigens, augumentation of a variety of cellular immune responses, synergistic activity with T-lymphocyte mitogens and increasing those immune responses depressed by disease or immunosupressive therapy.
The ability of levamisole to restore normal functions of phagocytes and T lymphocytes in compromised hosts has suggested its potential usefulness in immunotherapy2) for malignacy. Preliminary results in lung cancer and breast cancer has shown a survival gain in patients treated with levamisole3,4). Treatment with levamisole plus 5-fluorouracil reduced recurrence of colon cancer and death rate in patients with resected stage C colon cancer5). Furthermore, early reports indicated that side-effects were relatively uncommon and mild, leading to discontinuation of treatment in only 4.9% of 1179 colon cancer patients.
Effectiveness in the other types of tumors, including stomach cancer, has not been established.
We wish to report the results of a prospective, randomized, controlled study to determine the effectivenss of levamisole when it was added to conventional chemotherapy as an adjuvant therapy to surgery in patients with operable gastric cancer. The goals were to confirm the preliminary obervations of the disease free survival, overall survival and toxicity of levamisole.
From April 1991 to December 1992, 100 patients with gastric cancer who underwent curative resection in the Department of Surgery, University of Ulsan, Asan Medical Center, were assigned randomy to either group, one with or one without levamisole. Patient eligigibility was established at the time of the study entery.
The criteria for patients selection were as follows: 1) histologic documentation of adenocarcinoma of the stomach; 2) no privious treatment for cancer; 3)TNM stage IB or higher; 4) a potentially curative en bloc resection of tumors without gross or microcopic evidence of residual disease; 5) adequate bone marrow, renal and hepatic functions. Entry into the study was allowed no earlier than one week and no later than five weeks after surgery. All patients entered into the study gave informed consent and were randomly assigned to either chemotherapy with levamisole or chemotherapy alone. Both group had periodic evaluations. All patients were followed for total postoperative survival period. The patients attended the follow-up clinic at monthly intervals. On each visit, they were examined for evidence of recurrent tumor. Relevant investigatins were performed if tumor presence was suspected. Unless the side-effects were considered unacceptable, each treatment group received treatment for 6 months.
Hematologic testing and blood-chemistry panels were repeated every four weeks. The levamisol group received levamisole by mouth at a dose of 50mg every eight hours for a period of three days: this was repeated every two weeks for 6 months. Chemotherapy were consisted of 5-FU 1,000mg/m2/day administered as a continuous intravenous infusion for 5 days and cisplatin 60mg/m2/day as intravenous infusion for 1 day. This chemotherapy regimen repeated every 4 weeks for 6 cycles. Patients were required to have a white blood cell count (WBC) greater than 4,000/mm and a platelet counts(plateletes) greater than 150,000/mm. to receive therapy.
To receive midcycle of chemotherapy, the WBC had to be above 3,000/mm and platelets greater than 100,000/mm.
Criteria for recurrence included documentation by histologic or cytologic examination, or by evidence of metastases on the chest and bone x-ray.
Survival was the primary end point of this study. The time of the recurrence was also determined. Statistical analysis were carried out according to the procedures of the Statistical Analysis System. The survival curves were generated by the Kaplan-Meier method. The log-rank statistic was used to compare the distributions of survival times. The Cox propotional-hazards model wse used to determine the ratios of relapse and survival rates and to perform all multivariate analyses.
Out of 100 patients registered, fifty patients were assigned to each treatment group. There was no statistical difference in the distribution of ineligible, inevaluable, or dropout patients between the two groups.
The two groups were comparable in age, sex, tumor size, tumor location in the stomach, TNM stage and histologic differentiation(Table 1).
The majority of patients tolerated both treatment relatively well. Eleven patients in each gruop did not finish planned 6 courses of treatment due to non-complaiance. No patient withdrew from the study due to side-effect of the drug in both group. There was no significant difference in the side-effects of the drugs between chemotherapy alone and levamisole group. Mild nausea and vomiting (Grade II) were encountered in both treatment groups at the same rate (62 of 274 courses in the levamisole group and 55 of 260 courses in the chemotherapy alone group). Nausea, vomiting, leukopenia, fatigue, weakness were slightly more frequent in the levamisole group than in the chemotherapy alone group, but the difference was not statistically significant (p<0.05)(Table 2).
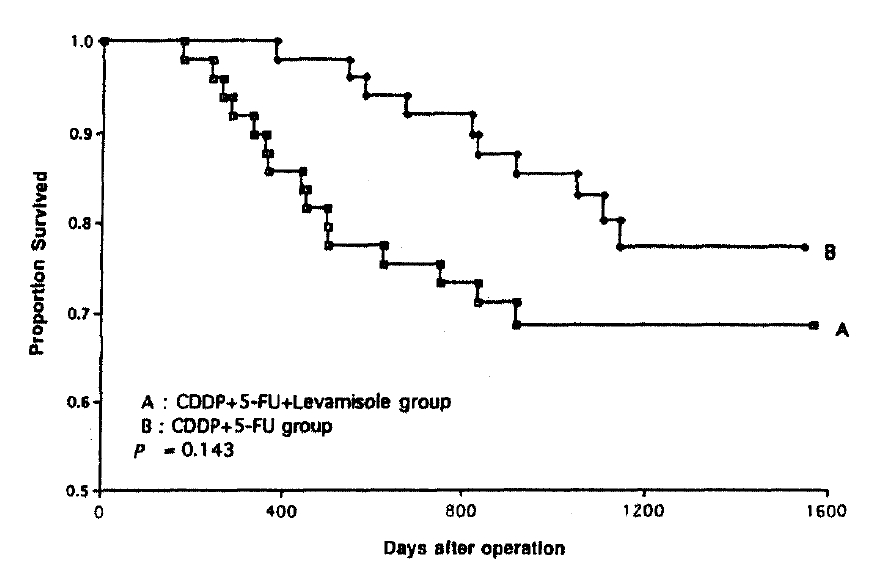
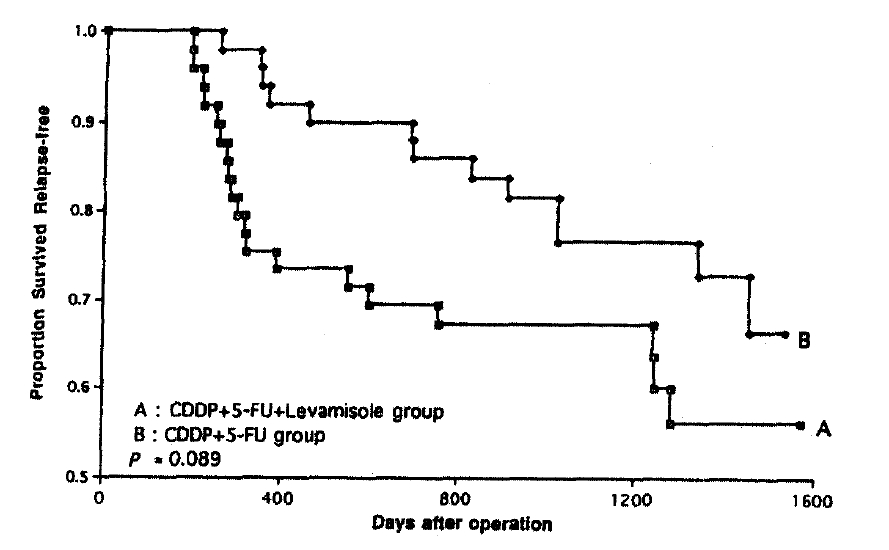
Table 3 shows the diseasefree survival and overall survival betwen the two groups. There was no significant difference in duration of survival between the two groups. At median follow up of 39 months, 32 patients relapsed with their disease. (19 in levamisle group and 13 in chemotherapy alone group(p=0.284)). 26 patients died after relapse, (16 in levamisole group and 10 in chemotherapy alone group)(p=0.077). Three patients expired without evidence of recurrence of gastric cancer in levamisole group. Listed causes of death were myocardial infarction (1 patient), sepsis (1 patient), and sudden death(1 patient). 4 patients in levamosloe group, 3 patients in chemotherapy alone group were alive with relapsed disease.
Among the patients with curative en bloc resction of gastric cancer, adjuvant chemotherapy with levamisole group increased the risk of overall death rate. At 3 years the levamisole-treated patients had a higher recurrence than the chemotherapy-only group. But these result was not significantly different between the levamisole and the chemotherapy alone groups.
Table 4 shows univariate analysis of disease-free survival and survival according to prognosis variables. In logistic regression using univariate analysis, chemoterapy with the levamisole group or the chemotherapy alone group(p=0.089), TNM stage (p=0.077) were trended to significant factors associated. In the analysis of survival and time to treatment failure, the key prognostic factors were the chemotherapy with or without levamisole and TNM stage. By a backward-regression selection procedure, chemotherapy, sex, age, tumor location, tumor size, N. stage, TNM stage and histologic grade were not all found to be independent determinants of recurrence(p>0.05). After correction for the influence of prognostic factors with Cox regression model, the difference in 3-year survival probablities of the levamisole group(69%) and chemotherapy alone group(83%) was 14%. But these result was not significant (p=0.143). Table 5 shows multivariate analysis of disease-free survival and survival according to prognostic variables by CoxŌĆÖs proportional harzards model. According to increased TNM stage (p=0.57, p=0.08) and after chemotherapy with levamisole rather than chemotherapy alone(p=0.15), the relative risk is increased.
Levamisole has been extensively evaluated in clinical trials for various cancer therapy, particuarly in the minimal disease as a adjuvant therapy after surgical treatment. There have been many reports showing a significant improvement in duration of remission and survival by actuarial analysis in the levamisole-treated group in the cases of cancers of the lung, colon and rectum5). The addition of levamisole to combination chemotherapy was found to increase both the response rate and survival in a randomized double-blind trial in patients with breast cancer and gastric cancer in one study6).
Another study failed to show a beneficial effect of additional levamisole7). In an experimental model system of KHT sarcoma in mice, the addition of levamisole did not improve local tumor control. It did not decrease the incidence of metastases8,9). Levamisole was found to have a negative influence on survival in a multihospital randomized trial in patients with extensive lung cancer. The does-related toxicity of levamisole was also found to be significant7). The results from a large series of 720 patients with breast cancer showed that the patients with N+M0 disease developed a significantly higher recurrence rate after total mastectomy with partially axillary lymph node biopsy when levamisole was combined with radiotherapy10). In malignant melanoma, the National Cancer Institute of Canada, Clinical Trials Group, conducted a similar study and reported benefit in the groups of patients receving levamisole11). However, in this same study, when levamisole was combined with BCG, no advantage whatever was obtained and in a comparable melanomaŌĆÖ adjuvant trial, Spittler et al found levamisole therapy to produce no advantage over placebo in either time to recurrence or survival12). In individual experiments with Moloney virus-induced rhabdomyosarcomas in BALB/c mice, levamisole treatmment seemed to be associated with an accelerated growth of the primary tumor, but this was not significant and even counter-balanced by a greater number of animals which showed inhibited tumor growth with levamisole7). The investigators, therefore, concluded that levamisole had no effect on the primary growth of these tumors. In an allogeneic model using L 1210 leukemia transplanted to C3H mice, an increased number of tumor takes was observed with levamisole treatment. This effect was associated with an elevated level of serum blocking activity and possible immunologic enhancement of tumor growth by levamisole. In light of the findings of Amery et al., levamisole is more effective when given prior to surgery. Japan Levamisole Research Association Group reported13), at operable gastric cancer patients receiving 600 mg Tegafur daily were then divided into two groups according to whether levamisole was given or not. The 2-year follow-up demonstrated that in stage III patients, the levamisole group was superior to the non-levamisole group, since the former prolonged the relapse-free interval significantly. The survival rate for stage III disease was also significantly higher in the levamisole group than in the non-levamisole group.
In our study, levamisole was added to the basic combination of cisplatin and 5-FU because the combination of cisplatin and 5-FU seems to be significantly more effective than the single agent treatment. However, in our trial, a poorer survival was found for en bloc resection of gastric cancer patients treated with levamisole than for non-levamisole groups. Our current findings would indication that the adjuvant use of levamisole to conventional chemotherapy in patients with gastric cancer after surgery did not provide an additional beneficial effect on duration or survival rate; in fact, in operable gastiric cancer patients, a negative influence on survival over a 3-year follow-up period was observed. In 1980, Brincker10) published his results of an interim analysis of a study very similar to ours and stated that levamisole was leading to a higher incidence of recurrences.
Our study shows no evidence of a favorable or unfavorable effect of levamisole on prognosis. The size of the study does not exclude the possibility of the existence of a small positive or negative effect. Our conclusion is that levamisole does not have any clinically significant influence on prognosis of patients with resectable gastric cancer. The both treatment arms were generally well tolerated and the toxicity profile was similar with or without levamisole.
Fig.┬Ā1.
Surval curves of patients with gastric cancer in all cases. Levamisole Versus Chemotherapy alone group.
Table┬Ā1.
Patient Characteristics
| CDDP+5-FU+levamisole (%) (n=50) | CDDP+5-FU(%) (n=50) | p-value* | |
|---|---|---|---|
| Age(years) | |||
| ŌĆā<55yr | 29(58) | 22(44) | 0.230 |
| ŌĆā>55yr | 21(42) | 28(56) | |
| Sex | |||
| ŌĆāMale | 34(68) | 39(78) | 0.386 |
| ŌĆāFemale | 16(32) | 11(22) | |
| Tumor location | |||
| ŌĆāUpper third | 7(14) | 5(10) | 0.575 |
| ŌĆāMiddle thrid | 7(14) | 11(22) | |
| ŌĆāLower third | 36(72) | 34(68) | |
| Tumor size(Cm) | |||
| ŌĆā<5.5 | 27(54) | 23(46) | 0.549 |
| ŌĆā>5.5 | 23(26) | 27(54) | |
| T stage | |||
| ŌĆāT1 | 2(4) | 1(2) | 0.687 |
| ŌĆāT2 | 4(8) | 4(8) | |
| ŌĆāT3 | 42(84) | 40(80) | |
| ŌĆāT4 | 2(4) | 5(10) | |
| N stage | |||
| ŌĆāN0 | 8(16) | 9(18) | 0.242 |
| ŌĆāN1 | 21(42) | 28(56) | |
| ŌĆāN2 | 21(42) | 13(26) | |
| TNM stage | |||
| ŌĆāIB | 2(4) | 1(2) | 0.510 |
| ŌĆāII | 8(16) | 11(22) | |
| ŌĆāIIIA | 18(36) | 22(44) | |
| ŌĆāIIIB | 22(44) | 15(15) | |
| ŌĆāIV | 0(0) | 1(2) | |
| Histologic grade | |||
| ŌĆāWell differentiated | 2(4) | 3(6) | 0.314 |
| ŌĆāMod. differentiated | 10(20) | 8(16) | |
| ŌĆāPoorly | 33(66) | 38(76) | |
| differentiated Undifferentiated | 5(10) | 1(2) | |
Table┬Ā2.
Side-effects by Treatment Arm
Table┬Ā3.
Disease Free Survival and Overall Survival*
| CDDP+5-FU+levamisole | CDDP+5-FU | P value* | |
|---|---|---|---|
| Total No. of patients | |||
| Patients with relapse | 50 | 50 | |
| ŌĆāLocal | 19 | 13 | |
| ŌĆāSystemic | 3 | 2 | |
| ŌĆāBoth | 10 | 8 | 0.284 |
| ŌĆāUnknown | 4 | 1 | |
| Patients who died | 3 | 2 | |
| ŌĆāNED#+died | 19 | 10 | |
| Relapse+died | 4 | 0 | |
| Patients who lived in relapse | 15 | 10 | |
| 4 | 3 | 0.077 | |
Table┬Ā4.
Univariate Analysis of Disease-free Survival and Survival According to Prognostic Variables
| Variable | No. of patients | NED | P-value* | Survival rate at 3yr | p-value* |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| Chemo+Leva | 50 | 67% | 0.089 | 69 | 0.143 |
| Chemo alone | 50 | 77% | 83 | ||
| Sex | |||||
| Male | 73 | 74% | 0.144 | 77 | 0.382 |
| Female | 27 | 66% | 74 | ||
| Age(years) | |||||
| ŌĆā<55yr | 51 | 70% | 0.247 | 74 | 0.914 |
| ŌĆā>55yr | 49 | 74% | 79 | ||
| Tumor location | |||||
| ŌĆāUpper third | 12 | 67 | 0.485 | 67 | 0.182 |
| ŌĆāMiddle thrid | 18 | 83 | 94 | ||
| ŌĆāLower third | 70 | 70 | 73 | ||
| Tumor size(Cm) | 0.189 | ||||
| ŌĆā<5.5 | 50 | 67 | 73 | 0.315 | |
| ŌĆā>5.5 | 50 | 76 | 79 | ||
| N. stage | |||||
| N0 | 17 | 87 | 0.176 | 87 | 0.161 |
| N1 | 49 | 74 | 79 | ||
| N2 | 34 | 61 | 67 | ||
| TNM stage** | |||||
| A | 22 | 81 | 0.077 | 81 | 0.065 |
| B | 40 | 79 | 84 | ||
| C | 38 | 60 | 64 | ||
| Histologic grade# | |||||
| Well & Mod. poorly & undiff | 23 | 78 | 0.938 | 83 | 0.783 |
| 77 | 70 | 74 | |||
Table┬Ā5.
Multivariate Analysis of Disease-free Survival and Survival According to Prognostic Variables by Coxs Proportional Hazards Model
REFERENCES
2. Stevenson Henry C, Green Ira, Hamilton J Michael, Calabro Barbara A, Parkinso R. Levamisole:Known Effects on the Immune System, Clinical Results and Future Applications to the Treatment of Cancer. J Clin Oncol 1991;9:2052ŌĆō2066.


3. Amery WK. Final results of a multicenter placebo-controlled levamisole study of resectable lung cancer. Cancer Treat Rep 1978;62:1677ŌĆō1683.

4. Kamby C, Rose C, Ejlertsen B, Andersen J, Brikler NE, Rytter LRytter, Andersen KW, Zedeler K. Adjuvant Systemic Treatment and the Pattern of Recurrences in Patients with Breast Cancer. Eur J Cancer Clin Oncol 1988;24:439ŌĆō447.


5. Mortel Charles G, Fleming Thomas R, Macdonald John S, Haller Daniel G, Laurie John A, Tangen Catherine M, Ungerleider James S, Emerson William A, Tormey Douglass C, Glick John H, Veeder Michael H, Mailliard James A. Fluorouracil plus Levamisole as Effective Adjuvant Therapy after Resection of Stage III Colon Carcinoma: A Final Report. Ann Intern Med 1995;122:321ŌĆō326.


6. Miwa Hiroaki, Ono Fumio, Moriyama Minoru, Kobayashi Tsutomu, Oka Tetsuhide, Nakamura Kenji, Orita Kunzo. Immunochemotherapy of gastric cancer with levamisole. Acta Med Okayama 1980;34(4):275ŌĆō281.

7. Davis Stephen, Mietlowski William, Rohwedder Jay J, Griffin John P, Neshat Amir A. Levamisole as an Adjuvant to Chemotherapy in Extensive Bronchogenic Carcinoma. Cancer 1982;50:646ŌĆō651.


8. Amery Willem K, Spreafico Federico, Rojas Alejandro F, Denissen Emiel, Chirigos Michael A. Adjuvant treatment with levamisole in cancer A review of experimental and clinical data. Cancer Treat Rev 1977;4:167ŌĆō194.


9. Baker D, Constable W, Elkon D, Rinehart L. The influence of ICRF-159 and levamisole on the incidence of metastses following local irradiation of a solid tumor. Cancer 1981;48:2179ŌĆō2183.


10. Executive Committee of the Danish breast Cancer Cooperative Group. Increased Breast-cancer Recurrence Rate After Adjuvant Therapy Wtih Levamisole; A preliminary Report. Lancet October 1980;16:824ŌĆō827.
11. Quirt Ian C, Shelly Wendy EShelly, Pater Joseph L, Bodurtha Audley J, McCulloch Peter B, McPherson Thomas A, Paterson Alexander HG, Prentice Robert, Silver Hulbert KB, Wilan Andrew R, Wilson Kenneth, Zee Benny. Improved Survival in Patients With Poor-Prognosis Malignant Melanoma Treated With Adjuvant Levamisole: A Phase III Study by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1991;9:729ŌĆō735.


12. Spilter LE. A randomized trial of levamisole versus placebo as adjuvant therapy in malignant melanoma. J Clin Oncol 1991;9:736ŌĆō740.


13. Niimoto Minoru, Hattori Takao, Ito Ichiji, Tamada Ryuichiro, Inokuchi Kiyoshi, Orita Kunzo, Furue Hisashi, Ogawa Nobuya, Toda Tomohiro, Furusawa Motonosuke, Koga Shigemasa, Hashimoto Isamu, Kondo Tatsuhei, Fujimoto Shigeru, Sugiyama Yuzuru, Abe Osahiko, Oya Masaaki. Levamisole in postoperative adjuvant immunochemotherapy for gastric cancer. Cancer Immunol Immunother 1984;18:13ŌĆō18.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



