INTRODUCTION
Biliary cystadenocarcinoma is a very rare cystic tumor and constitutes less than 5% of intrahepatic cysts of biliary origin1). Along with the recent advances in diagnostic imaging techniques, approximately 50 cases have been reported in the literature1ŌĆō13).
We report a case of biliary cystadenocarcinoma in a 63-year-old man with a review of the literature.
CASE REPORT
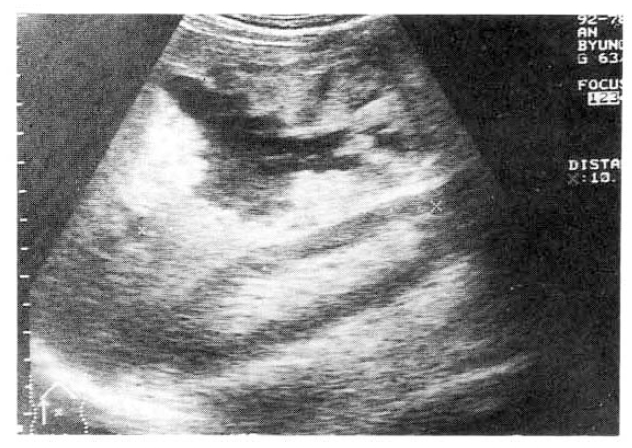
A 63-year-old man was readmitted to our hospital for further evaulation of hepatic cystic lesion. One year prior to admission, he had visited a local hospital due to pain in the right upper quadrant of the abdomen and fever. Abdominal sonographic finding showed about a 10cm-sized cystic nature space occupying lesion in the right lobe of the liver (Fig. 1) and pus-like material was aspirated. He was referred to our hospital for diagnosis and treatment. On admission, his temperature was 38.0┬░C, pulse 80 per minute, respiration 20 per minute. The blood pressure was 110/70mmHg. The laboratory tests were as follows: leukocyte, 12,900/mm3; hemoglobin, 12.9g/dl; total bilirubin, 4.6mg/dl; AST, 39IU/L; ALT, 74IU/L; Alkaline phosphatase, 590IU/L; GGT, 90IU/L. HBsAg and anti-HCV were all negative. Antiamebic antibody (IHA) was positive as 1:512 titer. Urinalysis and stool examination revealed within normal limits. The patient refused further study and was treated a as liver abscess. After discharge, he did not follow up with any specific problem for about 1 year. He visited to know the progress of the previous hepatic leision. He had anorexia and weight loss of over 1kg during 1 month. Upon readmission, his temperature was 37.0┬░C, pulse 80 per minute, respiration 20 per minute. The blood pressure was 120/80mmHg. There was no pathologic lesion in his eyes, ears, nasal or oral mucosa. On auscultation of chest, breathing sounds were normal, and the heart sound was regular without murmur. Three finger breadth palpable liver was felt on examination of the abdomen.
The results of a routine examination showed a hemoglobin of 13.3g/dl, hematocrit of 39%, a leukocyte count of 11,600/mm3 (neutrophil 67%, lymphocyte 27%, monocyte 6%) and a platelet count of 273,000/mm3. Liver function test showed as follows: total bilirubin, 0.9mg/dl; direct bilirubin, 0.6mg/dl; AST, 29IU/L; ALT, 13IU/L; alkaline phosphatase, 70IU/L; GGT, 38IU/L. Antiamebic antibody (IHA) was negative as below 1:64 titer. Urinalysis and stool examination revealed within normal limits. All tumor markers were within normal limits; AFP, 3.1ng/ml; CEA, 0.4ng/ml; CA 19-9, 8.96U/ml and CA 125, 40.1U/ml.
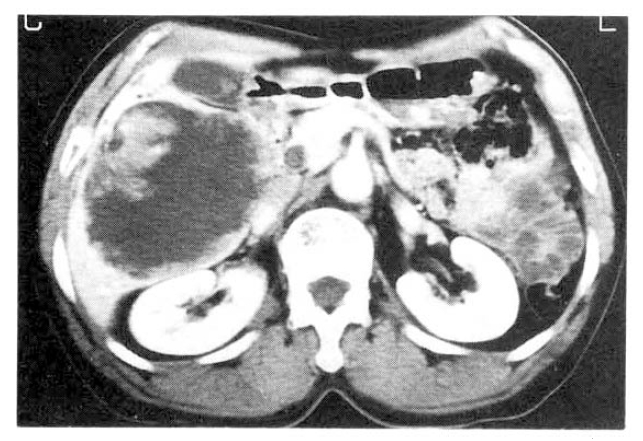
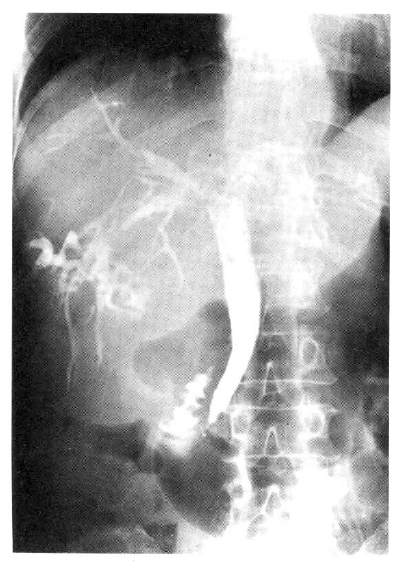
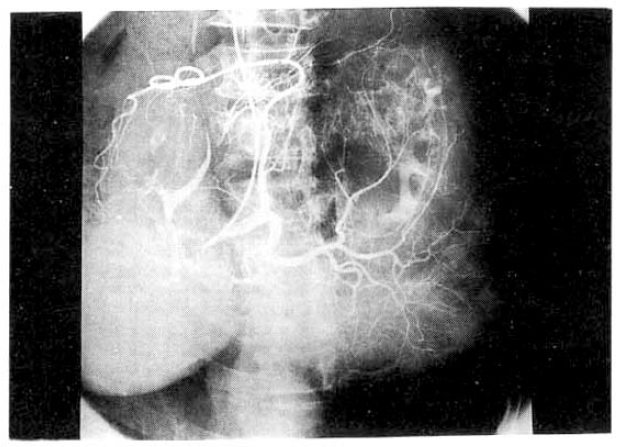
Chest and abdominal X-ray revealed no abnormality. Abdominal CT demonstrated huge cystic lesions measuring 10├Ś10cm in right liver and having focal irregular wall thickening with internal papillary projections (Fig. 2). Endoscopic retrograde cholangiography (ERCP) revealed mild dilated common bile duct and some filling defect in right intrahepatic duct. The cystic lesion was connected with right intrahepatic duct and some filling defect was seen in the cystic lesion (Fig. 3). Mucin-like material was drained by balloon catheter after sphincterotomy. Celiac angiography showed a large cavitary lesion which is supplied by hepatic artery (Fig. 4). Surgery was carried out for definitive diagnosis. On laparotomy, a huge hepatic mass was occupying in segment V and the cystic mass contained a portion of the right hepatic duct. The aspirated material from the cyst was found to be the same as the mucinous material drained endoscopically. He underwent right hepatic segmentectomy (segment V) with cholecystectomy.
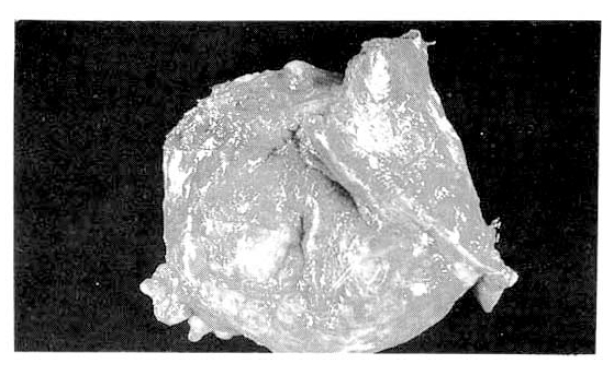
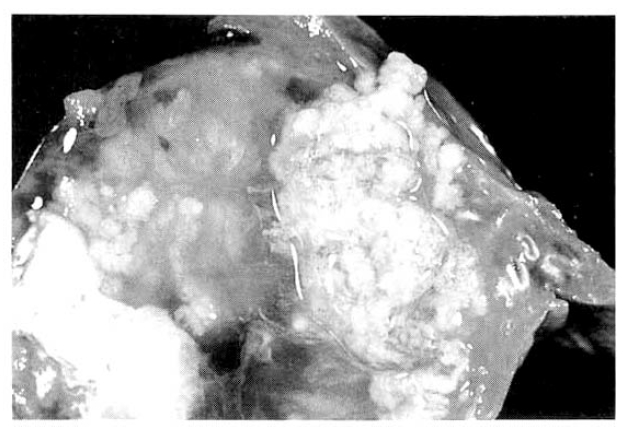
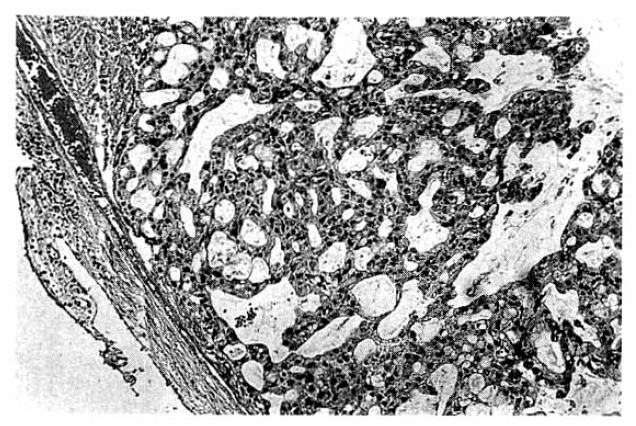
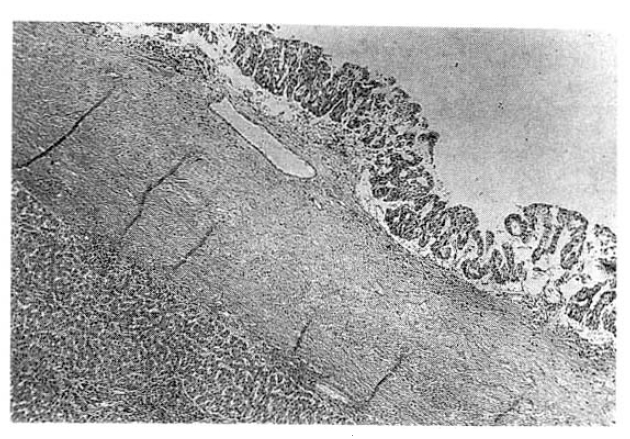
The surgical specimen contained a grayish-brown colored, well encapsulated unilocular cyst measuring 10.0├Ś8.0├Ś5.0cm (Fig. 5). On section, the internal cavity was filled with multiple papillary tumors and abundant mucinous fluid (Fig. 6). Histologic examination showed papillary proliferation of atypical cuboidal epithelium with mucin production in the cystic area. The tumor was determined as biliary cystadenocarcinoma (Fig. 7, 8).
The patient is doing well 2 year and 2 months after the operation and without a sign of recurrence.
DISCUSSION
Biliary cystadenocarcinoma is a very rare cystic tumor1) and it has been reported to arise from congenital liver cysts2ŌĆō9), bile ducts5, 6), biliary cystadenoma1ŌĆō12), fibropolycystic disease11) and hepatoduodenal ligament13). Approximately 50 cases have been reported in the literature1ŌĆō13). Sexual distribution is nearly equal but it is slightly more frequent in women14). The mean age at presentation is the late fifties. The tumor size ranges from 3cm to 26cm in the largest diameter and, even though some patients are asymptomatic, most of them are large in size accounting for the symptoms and sign, such as abdominal distention, nausea, palpable abdominal masses and abdominal local pain. Some patients show jaundice, fever, hemobilia and, occasionally, ascites.
However, with recent progress in imaging diagnosis, cystadenocarcinoma has been diagnosed with relative ease and the rapid enlargement of the tumor may be a possible sign of malignancy, but the clinical diagnosis is difficult during the preopeative course. The typical ultrasonographic appearance is that of a globular or ovoid thick-walled cystic mass. Fluid or low level echoes may be seen within the cyst, a sonographic appearance similar to that of pancreatic or ovarian cystadenomas or cystadenocarcinomas15). CT may demonstrate a multilobulated cystic mass that contains septations, papillary projections and, sometimes mural nodules16ŌĆō18). Our case was an unilocular cyst with internal papillary projections. ERCP and percutaneous transhepatic cholangiography demonstrate whether there are an intraluminal obstructing lesion and direct communication between the cyst lumen and the biliary tree, or not. In our case, ERCP showed that the cystic lesion was connected with the right hepatic duct and it suggests that the cyst has arisen in the bile duct. Angiography demonstrates a hypovascular mass in most instances or, sometimes, a small vascular blush along the periphery of the tumor as in our case 19).
Grossly, the tumor is usually large-size and consists of a solitary, well encapsulated, fluid-containin multilocular cyst. The cyst often contains hemorrhagic, bilous, mucinous or clear fluid. Wee et al12) reported a case of biliary adenocarcinoma which was diagnosed preoperatively based on the fine needle aspiration cytologic findings and the imaging appearances. But, the results are usually negative and the chance of peritoneal seeding of malignant cells is present with such a procedure.
Histopathologically, the epithelium lining the cystic spaces is mostly nonciliated columnar to cuboidal mucin secreting epithelium and portions of transition from cystadenoma to cystadenocarcinoma tumor cells may be seen. Most epithelial tumor cells are positive on immunohistochemical staining with antibodies to cytokeratin, epithelial membrane antigen and carcinoembrionic antigen14). Biliary cystadenocarcinoma must be differentiated from benign biliary cystadenoma, cholangiocarcinoma and metastatic adenocarcinoma. It needs complete surgical resection for definitive diagnosis and treatment.
The prognosis is comparatively good with complete resection and generally seems to be much better than that for intrahepatic bile duct carcinoma1). Recently, Devaney et al14) recognized that there was a close relation between morphological findings and prognosis. Two types of cystadenocarcinoma exist. One is seen in female patients and arise from a preexisting biliary cystadenoma, usually accompanied by ovarian stroma, and follows an indolent course. The other is found in the male, not associated with a preexisting biliary cystadenoma with ovarian stroma, is more aggressive and leads to death in over half the patients14).
In conclusion, we report here a rare case of biliary cystadenocarcinoma in a 63-year-old man who was treated for liver abscess, although our case was unilocular, which is not typical.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





