INTRODUCTION
Thyrotropin-releasing hormone (TRH) is a tripeptide that was originally characterized in extracts of ovine and porcine hypothalamus1). Much of the hypothalamic TRH is localized within the pavocellular subdivision of the paraventricular nucleus. Cells in this region project to median eminence to regulate anterior pituitary thyrotropin secretion. TRH is also present in many extrahypothalamic loci and plays roles as a neurotransmitter and/or a neuromodulator in the central nervous system and in the gastrointestinal tract2ŌĆō4). In 1986, Lechan et al. isolated a nearly full length TRH cDNA from a rat hypothalamic bacteriophage Igt11 expression library5) which allowed investgations of the regulation of TRH mRNA levels in intact animals.
Evidence for a direct effect of glucocorticoids on the TRH :gene expression in the paraventricular nucleus (PVN) of the hypothalamus include the presence of glucocorticoid receptors in the PVN6) and the decreased TRH gene expression by glucocorticoid treatment in the rat PVN shown by in situ hybridization of Lechan and co-workers7). In contrast to the rat TRH gene expression in the PVN, glucocorticoid treatment stimulated the expression of extrahypothalamic rat TRH gene. Glucocorticoid treatment increasd the TRH mRNA in the murine neoplastic medullary thyroid carcinoma cell line (CA77)8) and the fetal diencephalic neuron9ŌĆō10). It also increased a parallel rise of TRH, pre-pro-TRH-(25ŌĆō50) and pro-TRH mRNA in the somatotrophs of long-term cultured rat anterior pituitary gland11), indicating that the effect probably did not occur at the post-translational level. Furthermore, Rondeel et al. suggested that dexamethasone (DEX) did not alter pro-TRH mRNA stability in the rat anterior pituitary gland12). Therefore, one might speculate that glucocorticoid regulates the TRH gene expression at the transcriptional level.
The mechanism by which glucocorticoid stimulates gene transcription has been studied in detail. In the absence of hormone, glucocorticoid receptor (GR) is located in the cytoplasmic space in association with a protein called heat-shock protein 90 (hsp90). When the concentration of glucocorticoid in the extracellular medium is increased, the hormone moves across the plasma membrane into the cytoplasmic space where it bidns to the GR and induces dissociation of the GR-hsp90 complex and dimerization of the GR. The resulting complex of hormone and GR moves into the nucleus through nuclear pores. Within the nucleus, the glucocorticoid-GR complex binds to the glucocorticoid response element (GRE) on a target gene13,14). The consensus sequence of the GRE has been revealed to be located within a few hundred base pairs of the transcription start site of hormone responsive genes, and has a palindromic structure known as GAACAnnnTGTTC15).
The discrepancy between glucocorticoid-induced regulation of the TRH gene expression in the PVN and extrahypothalamic sites suggests that the diversity of response might be based on the interaction betwen a cell-specific transcription factor(s) and GRE. However, the GRE of the rat TRH gene has not been defined, to date. As the first step to understand the molecualr: mechanism for the glucocorticoid regulation of the TRH gene expression, we tried to identity the GRE on the promoter of the rat TRH gene.
MATERIALS AND METHODS
1. Materials
HeLa cell was purchased from American Type Culture Collection. DulbecoŌĆÖs modified Eagles Medium (DMEM), fetal calf serum (FCS), penicillin and streptomycin and trypsinwere obtained from Gibco-BRL. Dexamethasone, O-nitrophenyl-D-galactopyranoside (ONPG), adenosine triphosphate (ATP) and D-luciferin were obtained from Sigma. Calf intestinal alkaline phosphatase, T4 kinase, T4 ligase, Klenow fragments and all restriction enzymes were purchased from Promega. Reagents for syntheitic oligonucleotides were supplied by Applied Biosystem. All the other chemicals were purchased from Promega and BRL.
2. Plasmids
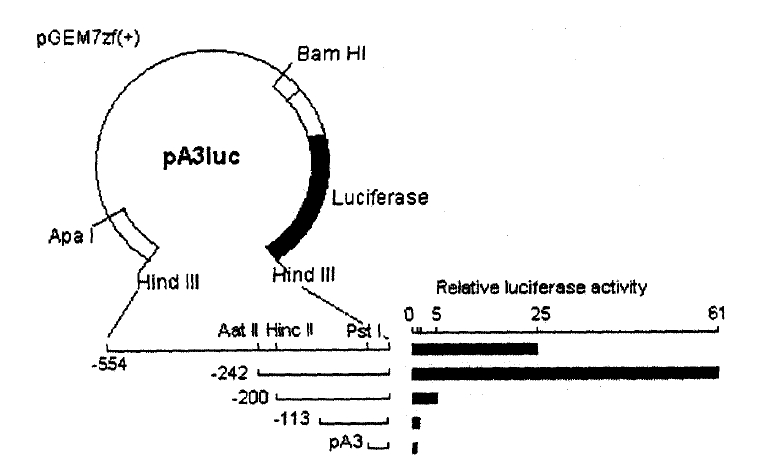
For the 5ŌĆ▓ deletion study of the TRH gene, six different plasmid constructs, pTRH(ŌłÆ554/84)-LUC, pTRH(ŌłÆ242/84)-LUC, pTRH(ŌłÆ200/6)-LUC, pTRH(ŌłÆ113/84)-LUC, pTRH(ŌłÆ77/84)-LUC and pTRH(ŌłÆ47/84)-LUC, were used for transient gene expression. Each plasmid was constructed by subcloning the serially truncated 5ŌĆ▓ upstream regulatory region of the rat TRH gene at the position of ŌłÆ554, ŌłÆ242, ŌłÆ200, ŌłÆ113, ŌłÆ77, ŌłÆ47 into the promoterless luciferase expression vector, pA3LUC16,17), which was generously provided by Dr. W. Wood. All of the plasmid construct were verified by sequencing.
The plasmid pMAMneo-LUC was used as a positive control plasmid for GRE. The plasmid contains the mouse mammary tumor virus promoter, including multiple copies of GRE18), which was kindly provided by Dr. B. Sandboun.
The plasmid pRSV-GR, provided by Dr. K. Yamamoto, was a plasmid vector that highly expresses GR. It includes a full length GR cDNA which was subcloned downstream to the promiscous Rous Scarcoma Virus (RSV) promoter19).
3. Transfection
Monolayer culture of HeLa ceils was maintained in DMEM supplemented with 10% fetal calf serum and penicillin-streptomycin at 37┬░C under 5% CO2. HeLa cells were cultured in 100 mm dishes for 1ŌĆō2 dyas until 60% confluent before transfection. Transfection was performed by the calcium phosphate precipitation method20) with 2 ╬╝g of pMAM-LUC (MMTV-LUC) as a positive control or 2 ╬╝g of each of the pTRH-LUC vectors described above, 2 ╬╝g of the GR expression vector (pRSV-GR) and 1 ╬╝g of b-galactosidase gene. Fourhovrs after transfection, the cells were washed once with phosphate buffered saline (PBS) and treated with 15% glycerol for 1 min. Afterwards, cells were washed twice with PBS and briefly treated with trypsin before replating in 65 mm dishes. After 24 hrs of incubation, the medium was replaced with fresh medium varing concentrations of dexamethasons (DEX) and the cells were cultured for another 24 hrs. The cellular extracts were subjected to the luciferase assay and ╬▓-galactosidase assay for control of transfection efficiency.
RESULT
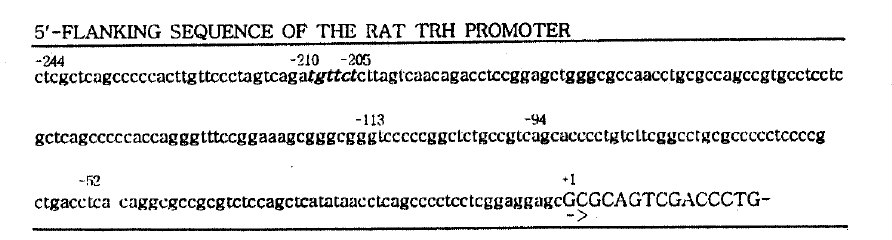
A single perfect GRE half-site, TGTTCT, was found between 205 and 211 bp upstream of the transcription start site of the rat TRH gene21) (Fig. 1). To confirm whether the GRE half-site confers the glucocorticoid responsiveness, we examined the transcriptional stimulation of the luciferase gene by the various 5ŌĆ▓ upstream regions of TRH gene.
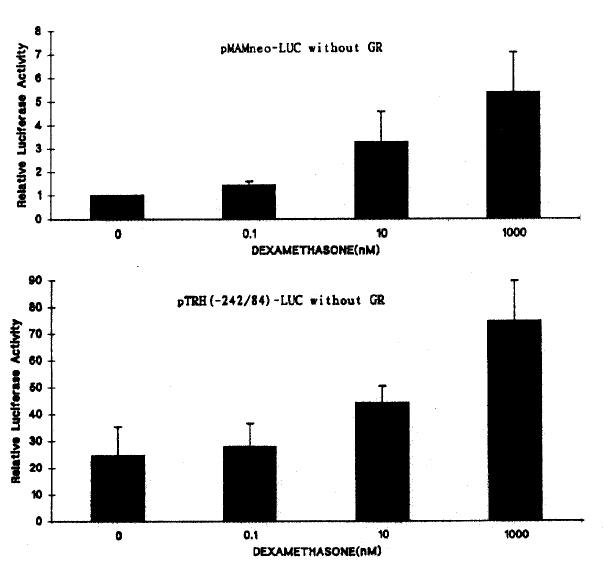
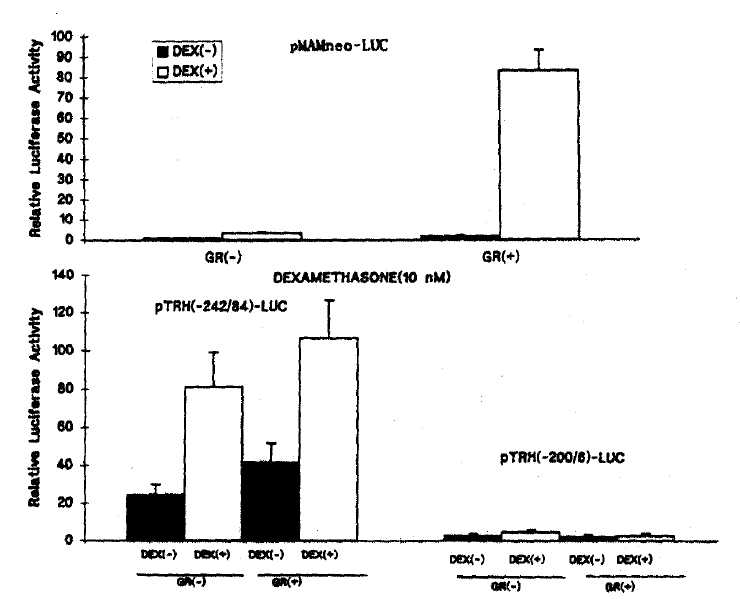
The basal transcription of pMAMneo-LUC, a positive control for GRE, was only about 3.2 fold stimulated by DEX at the physiologic concentration of 10ŌłÆ8 M and 5.4 fold at 10ŌłÆ6 M (Fig. 2). However, co-transfection of GR expression vector (pRSV-GR) enhanced the DEX-stimulated transcriptional activity of pMAMneo-LUC by approximately 83 fold (Fig. 4).
DEX also stimulated the transcriptional activity of pTRH(ŌłÆ554/84)-LUC and pTRH(ŌłÆ242/84)-LUC by apprximately 1.8 fold (p < 0.01) at 10ŌłÆ8 M and 3.1 fold (p < 0.01) at 10ŌłÆ6 M. On the contrary, deletion of the region between ŌłÆ242 to ŌłÆ200 bp reduced the basal transcriptional activity by 90% and also completely abolished the DEX-induced transcriptional activation of the luciferase gene (Fig. 3, 4). The DEX-induced transcriptional activation of pTRH(ŌłÆ242/84)-LUC was in dose-dependent manner (Fig. 2). These findings suggest that a cis-acting element(s) which is important for the basal transcriptional activation and glucocorticoid response of the rat TRH gene is located between ŌłÆ242 bp and ŌłÆ200 bp.
While the co-transfection fo pRSV-GR did not increase the basal transcriptional activity of pMAMneo-LUC, it increased the basal transcription of pTRH(ŌłÆ242/84)-LUC by 1.8 fold (p < 0.05). The pRSV-GR cotransfection and DEX treatment further increased the transcription of pTRH(ŌłÆ242/84)-LUC by 2ŌĆō4 fold at the concentration of 10ŌłÆ8M.
DISCUSSION
It was suggested that glucocorticoid exerts cell-specific effects on TRH gene expression at the transcriptional level12).
Although there is no typical palindromic GRE on the rat TRH gene promoter, a perfect GRE half-site was found between ŌłÆ205 and ŌłÆ210 bp. Our result shows that deletion of the segment between ŌłÆ242 and ŌłÆ200 bp of the rat TRH promoter abolishes DEX-induced transcriptional activation as well as the basal transcriptional activation. DEX increased the transcriptional activation of pTRH(ŌłÆ242/84)-LUC in dose-dependent manner. It suggests that the segment contains on important element(s) for the glucocorticoid response as well as basal transcriptional activation. In fact, Stevenin et al. recently demonstrated that the DNA binding domain of GR binds tightly to the region between ŌłÆ220 and ŌłÆ200 bp of the rat TRH promoter by using DNase I footprinting22), which supprts the present result.
Although the transcription of pMAMneo-LUC as a GRE positive control vector was supposed to be stimulated more than 10 fold by DEX, it was, in fact, stimulated only about 5.3 fold by DEX without GR co-transfection in the present study. GR co-transfection, however, dramatically increased the response by 83 fold. It suggests that HeLa cell is deficient of GR. Thus, the possibility can not be excluded that the DEX-induced transcriptional activation of pMAMneo-LUC was mediated by other transcriptional factor(s) induced by DEX. Without GR co-transfection, the transcription of pTRH(ŌłÆ242/84)-LUC was also stimulated to a similar degree by DEX, as was pMAMneo-LUC. Accordingly, the DEX-induced transcriptional activation of pTRH(ŌłÆ242/84)-LUC also could be mediated by other DEX-inducible transcription factor(s).
The basal transcription of pTRH(ŌłÆ242/84)-LUC was approximately 1.8 fold increased by co-transfection of GR, wherease that of pMAMneo-LUC was not stimulated at all. It suggest that the increased basal transcriptional activity may result from an interaction between GR and other transcription factors that are involved in the basal and hormonal regulation of transcription. In fact, earlier reports demonstrated that there are elements for the binding of cAMP response element binding protein (CREB), c-Jun and thyroid hormone receptor between ŌłÆ47 and ŌłÆ113 bp23,24,25). HeLa cell is known to express CREB and c-Jun/c-Fos26). Thus, it is possible that GR may interact with other transcription factor(s) including CREB and c-Jun/c-Fos, and which, in turn, enhance basal transcriptional activity.
With DEX stimulation, the increased basal transcriptional activity was further enhanced by 2.6 fold. The magnitude was remarkably smaller than that of pMAMneo-LUC and similar to that without DEX stimulation. It suggests two possibilities. One is that the GRE of the rat TRH gene is much weaker than that of pMAMneo-LUC. As pMAMneo-LUC has multiple copies of GRE, it is known to show very strong response to glucocorticoid. On the contrary, there is only a half-site of GRE on the TRH gene. This could make the difference in the degree of the glucocorticoid response.
The other possibility is that the glucocorticoid effect on the transcription is mediated through the activation of other transcriptional factor(s). Synergistic effect of glucocorticoid and activators of the protein kinase A pathway on the enkephalin gene transcription have been previosly demonstrated27). The potentiating effects of two regulators of transcriptional activity may derive from multiple mechanisms, most likely involving the action and/or interaction of transcriptional modulators of the proto-oncogene families28). TRH and c-Jun/c-Fos are co-expressed in cultured rat anterior pituitary cells and their expressions are enhanced by DEX in a similar fashion at the transcriptional level29,30). Also, the TRH genes are expressed in somatotroph of anterior pituitary gland31). Jackson et al. proposed three possible mechanism. Firstly, DEX stimulates TRH gene expression by enhancing c-fos/c-Jun gene expression. Secondly, DEX initially enhances the expression of c-Fos/c-Jun which induces other transacting factors, and which, in turn, increase TRH gene expression. Finally, the gene expressions of TRH and c-fos/c-Jun are stimulated separately by DEX and then c-Fos/c-Jun is responsible for prolonging the expression of the TRH gene rather than initiation TRH gene expression. We, therefore, hypothesize that the region between ŌłÆ242 and ŌłÆ200 bp of TRH promoter is important for potentiation of transcriptional activity in association with other proto-oncogene families. In conclusion, the sequences between ŌłÆ242 bp and ŌłÆ200 bp of the rat TRH gene promoter contain a weak glucocorticoid response element. The glucocorticoid response seems to be mediated by an interaction between glucocrticoid receptor and other transcriptional factor(s).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





