 |
 |
| Korean J Intern Med > Volume 11(2); 1996 > Article |
|
Abstract
Objectives
The prognosis of IDDM is mainly dependent on complicated diabetic nephropathy which is probably determined by both metabolic abnormalities and genetic predisposition. Angiotensin I converting enzyme (ACE) regulates systemic and renal circulations through angiotensin II formation and kinins metabolism. The insertion(I)/deletion(D) polymorphism in intron 16 of ACE gene is strongly associated with ACE levels, and subjects homozygote for deletion (genotype DD) have the highest plasma values. Recently, it was reported that I/D polymorphism of ACE gene is associated with diabetic nephropathy in Caucasian IDDM patients. We studied the relationship between the ACE gene polymorphism and diabetic nephropathy in Korean IDDM patients.
Methods
The study population consisted of 59 IDDM patients (duration> 5yrs) and 107 control subjects. IDDM subjects were divided into 2 groups according to the presence or absence of diabetic nephropathy (with nephropathy: n=31, without nephropathy: n=28). After extraction of genomic DNA from peripheral blood leukocytes, PCR was performed using the sense primer (5ŌĆ▓-GCC CTG CAG GTG TCT GCA GC-3ŌĆ▓) and anti-sense primer (3ŌĆ▓-TGC CCA TAA CAG TGC TTC ATA-5ŌĆ▓), respectively. The PCR products were electrophoresed in 2% agarose gels, and DNA was visualized directly with ethidium bromide staining.
Results
Frequencies for II, ID and DD genotypes were similar in IDDM subjects and controls (23:19:17 vs 49:41:17, p=0.142) and derived allele frequencies for I and D alleles were similar in both groups (0.551:0.449 vs 0.649:0.351, p=0.098). The ACE genotype distributions were not different in diabetic subjects with or without nephropathy (12:9:10 vs 11:10:7, p=0.78) and derived allele frequencies were also similar (0.532:0.468 vs 0.571:0.429, p=0.81).
Conclusion
The I and D allele frequency in our controls was different compared to ACE allele frequencies of Caucasian populations, but very similar compared to those of Chinese or Japanese subjects. We found that I/D polymorphism of ACE gene is not implicated in the diabetic nephropathy of Korean IDDM patients and may be explained by ethnic differences.
Key words
Insertion/Deletion polymorphism; ACE gene; IDDM; Nephropathy; Ethnic DifferencesDiabetic nephropathy is associated with the greatest morbidity and mortality in IDDM1). Although the vast majority of diabetic patients have some degree of retinopathy, nephropathy develops in only 35 to 45% of patients with IDDM2), so there is probably a genetic basis for diabetic nephropathy. Previous reports of clustering of diabetic nephropathy in families also provide support for the hypothesis that genetic factors contribute to susceptibility to diabetic nephropathy3,4).
Angiotensin I converting enzyme (ACE) is a dipeptidase, which has a key role in regulating systemic and renal circulations, activating angiotensin I into the vasoconstrictor peptide angiotensin II, and inactivating the vasodilatory peptide bradykinin5). The ACE gene is 21 kilobases long on the chromosome 17 and consists of 26 exons6). A genetic polymorphism has been described in intron 16 of the ACE gene7). Individuals varied as to the presence (insertion, I) or absence (deletion, D) of a 287 fragment. It has been estimated that the I/D polymorphism accounts for about 47% of the total phenotype variance of serum ACE activity in the normal Caucasian population8). Subjects homozygote for the deletion (DD) display the highest values and those homozygote for the insertion (II) display the lowest, with heterozygotes displaying intermediate values. An excess of the DD genotype was recently observed in subjects with myocardial infarction9), left ventricular hypertrophy10) and idiopathic dilated cardiomyopathy11) which suggests that a genetic polymorphism affecting ACE gene expression could be associated with susceptibility to vascular disease.
In Caucasian diabetic patients, it was reported that serum ACE activity is especially elevated in IDDM subjects with microalbuminuria12) and II genotype of ACE gene is a marker for reduced risk for diabetic nephropathy13). However, I/D allele frequency of ACE gene is known to be different between Caucasian and Oriental normal populations14,15). The present study was performed to see whether I/D polymorphism of ACE gene is associated with diabetic nephropathy in Korean IDDM patients.
The study population consisted of 59 IDDM patients who had diabetic duration for more than 5 years and 107 control subjects. IDDM subjects were divided into 2 groups according to the presence or absence of diabetic nephropathy: 1) normoalbuminuric subjects (n=28) who are IDDM patients with an albumin excretion rate (AER)<20 ug/min and 2) nephropathy cases (n=31) who have microalbuminuria or overt proteinuria or end-stage renal disease (ESRD). Microalbuminuria was defined by urinary AER between 20ŌĆō200 ug/min in the absence of permanent hypertension. Overt proteinuria was defined by 24 hour urinary protein excretion of more than 500 mg. Cases with nephropathy consisted of 9 subjects with microalbuminuria, 19 with overt proteinuria and 3 with ESRD. Diabetic retinopathy was classified by independent opthalmologists as zero, background, preproliferative, proliferative.
DNA was extracted from peripheral blood leukocytes by the salting out method. The sequence of the sense primer and anti-sense primer were 5ŌĆ▓-GCC CTG CAG GTG TCT GCA GC-3ŌĆ▓ and 3ŌĆ▓-TGC CCA TAA CAG TGC TTC ATA-5ŌĆ▓, respectively. PCR was performed in a final volume of 40 ╬╝l which contained 1.0 ug of genomic DNA, 10 pmol of each primer, 200 uM each of the. four dNTP, 1.5 mM MgCl2, 40 mM KCl, 10 mM Tris-HCl, pH 8.0 and 2.0 unit of Taq polymerase (Korea Biotech. Inc, Taejeon, Korea). Amplication was carried out in a thermocycler (Perkin Elmer Cetus, UK) for 35 cycles with steps of denaturation at 94┬░C for 1 min, annealing at 60┬░C for 1 min and extension at 72┬░C for 2 min. The PCR products were electrophoresed in 2% agarose gels, and DNA was visualized directly with ethidium bromide staining.
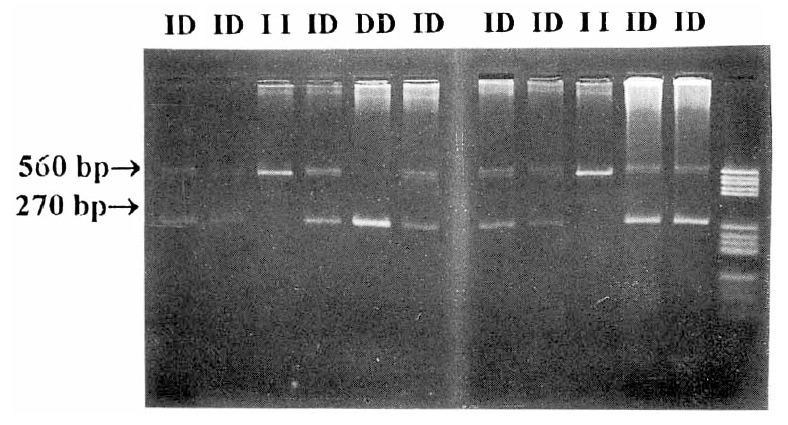
The insertion/large allele (560 bp) is designated I, and the deletion/shorter allele (270 bp) is designated D. Thus, each DNA sample yielded one of three possible genotypes represented as II, ID, and DD (Fig. 1). Frequencies for II, ID and DD genotypes were 23, 19 and 17 in IDDM patients, and 49, 41 and 17 in control subjects, respectively. Derived allele frequencies for I and D alleles were 55.1% and 44.9% in IDDM patients and 64.9% and 35.1% in control subjects, respectively, indicating that there was no significant difference between the two groups (Table 1). In IDDM patients, the subjects with proliferative diabetic retinopathy (PDR) were 11 patients. Frequencies for genotypes of ACE gene were 3, 4 and 4 in subjects with PDR and 20, 15, 13 in subjects without PDR, and derived allele frequencies for I and D alleles were 45.5% and 54.5% in subjects without PDR and 57.3% and 42.7% in subjects without PDR. There was no significant difference between subjects with or without PDR (Table 2).
Talbe 3 gives the clinical characteristics of 31 IDDM cases with nephropathy and 28 IDDM subjects without nephropathy. There was no significant difference in onset age, duration of diabetes and HbAlc level. But the subjects without nephropathy showed a tendency to have a mild degree of retinopathy. Frequencies for II, ID and DD genotypes were 12, 9 and 10 in nephropathy cases and 11, 10 and 7 in cases without nephropathy, and derived allele frequencies for I and D alleles were 53.2% and 46.8% in nephropathy cases and 57.1% and 42.9% in cases without nephropathy. There was no significant difference between IDDM patient with or without nephropathy (Table 4).
The findings of previous epidemiological and family studies suggest that diabetic nephropathy results from an interaction between metabolic abnormalities that are typical of poorly controlled IDDM and predisposing genetic factors3,4). The nature of the genetic factors, however, has remained unknown. ACE is the component of the renin angiotensin system that catalyzes its inactive precursor angiotensin I. Angiotensin II affects vasoconstriction and sodium retention, alters kidney hemodynamics and increases systemic blood presure16). A likely possibilty is that the ACE variant acts as a risk factor for diabetic nephropathy by modulating renal hemodynamics without affecting systemic blood pressure, as has been hypothesized for myocardial infarction9). In the kidney, ACE mRNA is in endothelial, mesangial and epithelial cells6). Other than activating conversion of angiotensin I to angiotensin II, ACE is a component of the intrarenal kallikrein-kinin system, being an inactvatior of bradykinin16). These two peptides, angiotensin II and bradykinin, have opposite effects on renal circulation. Recently, Marre at al13) suggested that II genotype of ACE gene is a marker for reduced risk for diabetic nephropathy in white French IDDM subjects, and Doria et al17) reported that DNA sequence differences in the ACE gene may contribute to genetic susceptibility to diabetic nephropathy in Caucasian IDDM subjects. Because IDDM has many underlying causes that may be specific to the genetic and cultural background of the patient, racial differences in genetic and etilogical mechanisms are of interest. In the present study, no association was demonstrated, indicating that ACE gene polymorphism is not implicated in the developement of diabetic nephropathy in Korean IDDM patients. It is noteworthy that the frequency of I allele in our normal control subjects, 0.649, was higher than the previously reported values of 0.431 by Tiret et al7) and 0.406 by Rigat et al8) and was very similar to the value of 0.701 by Lee14) in Chinese and 0.601 by Higashimori et al15) in Japanese control subjects. Although these differences may be due to selection bias of normal control subjects, it is most likely to be due to ethnic difference. Contrary to the previous reports, Tarnow et al18) reported that I/D polymorphism of ACE gene does not act as a risk factor for the development of diabetic nephropathy in Danish IDDM subjects.
We observed that I/D polymorphism of ACE gene is not implicated in the diabetic nephropathy of Korean IDDM patients. Although our sample size is small, our result may be explained by ethnic difference. If the genetic approach has provided new insights into the pathogenesis of diabetic nephropathy, studies involoving various ethnic groups are important.
Acknowledgments
This study was supported by grants from Seoul National University Hospital (02-94-285) and was presented in part at the 1994 Annual Meeting of the Korean Diabetes Association in Seoul, Korea.
We thank Dr. Hyo Soo Kim (Seoul National University Hospital, Seoul, Korea) for providing advice of the primer design.
Fig.┬Ā1.
Examples of the 3 patterns of the ACE insertion/deletion polymrphism. Homozygotes (II) have a band of 560 pb, homozygotes (DD) have a band of 270 pb and heterozygotes (ID) have both bands. The last lane shows size marker (Hae III).
Table┬Ā1.
Frequencies of I/D Polymorphism of ACE Gene in IDDM (n=59) and Control Subjects (n=107)
| IDDM | Controls | |
|---|---|---|
| Genotypes | ||
| II | 23(39.0%) | 49(45.8%) |
| ID | 19(32.2%) | 41(38.3%) |
| DD | 17(28.8%) | 17(15.9%) |
| Alleles | ||
| I | 65(55.1%) | 139(64.9%) |
| D | 53(44.9%) | 75(35.1%) |
Table┬Ā2.
Frequencies of I/D Polymorphism of ACE Gene in IDDM Patients with or without PDR
| PDR(+) (n=11) | PDR(ŌłÆ) (n=48) | |
|---|---|---|
| Genotypes | ||
| II | 3(27.2%) | 20(41.7%) |
| ID | 4(36.4%) | 15(31.2%) |
| DD | 4(36.4%) | 13(27.1%) |
| Alleles | ||
| I | 10(45.5%) | 55(57.3%) |
| D | 12(54.5%) | 41(42.7%) |
Table┬Ā3.
Clinical Characteristics of Study Subjects
REFERENCES
1. Hostetter TH. Diabetic nephropathy. The Kidney. 4th ed. In: Brenner BM, Rector FC, eds. Philadelphia: Saunders, 1991;1695ŌĆō1727.

2. Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type I (insulin-dependent) diabetes: an epidemiologic study. Diabetologia 1983;25:496ŌĆō501.


3. Sequist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to deabetic nephropathy. N Engl J Med 1989;320:1161ŌĆō1165.


4. Quinn M, Angelico MC, Cross A, Gearin G, Warram JH. Concordance for kidney complications in siblings with IDDM (abstract). Diabetes 1992;41(suppl. 1):121A.
5. Erdos EG. Angiotensin I-converting enzyme and the changes in our concepts through the years. Hypertension 1990;16:363ŌĆō370.


6. Hubert C, Houot A-M, Corvol P, Soubrier F. Structure of the angiotensin I-converting enzyme gene: Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem 1991;266:15377ŌĆō15383.


7. Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I converting enzyme (ACE) gene controls plasma ACE activity. Am J Hum Genet 1992;51:197ŌĆō205.


8. Rigat B, Hubert C, Alhence-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990;86:1343ŌĆō1346.



9. Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, Luc G, Bard JM, Bara L, Ricard S, Tiret L, Amouyel P, Alhenc-Gelas F, Soubrier F. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 1992;369:641ŌĆō644.

10. Schunkert H, Hense HW, Holmer SR, stender M, Perz S, Keil U, Lorell BH, Riegger GAJ. Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertophy. N Engl J Med 1994;330:1634ŌĆō1638.


11. Raynolds MV, Bristow MR, Bsuh EW, Abraham WT, Lowes BD, Zisman LS, Taft CS, Perryman MB. Angiotensin-converting enzyme DD genotype in patients with ischemic or idiopathic dilated cardiomyopathy. Lancet 1993;342:1073ŌĆō1075.


12. Hallab M, Bled F, Ebran JM, Suraniti S, Girault A, Fressinaud PH, Marre M. Elevated serum angiotensin converting enzyme activity in type I, insulin-dependent diabetic subjects with persistent microalbuminuria. Acta Diabetol 1992;29:82ŌĆō85.

13. Marre M, Bernadet P, Gallois Y, Savagner F, Guyene TT, Hallab M, Cambien F, Passa P, Alhenc-Gelas F. Relationship between angiotensin I converting enzyme gene polymorphism, plasma levels and diabetic retinal and renal complications. Diabetes 1994;43:384ŌĆō388.


14. Lee EJD. Population genetics of the angiotensin-converting enzyme in Chinese. Br J Clin Pharmac 1994;37:212ŌĆō214.



15. Higashimori K, Zhao Y, Higaki J, Kamitani A, katsuya T, Nakura J, Miki T, mikami H, Ogihara T. Association analysis of a polymorphism of the angiotensin converting enzyme gene with essentiall hypertension in the Japanese population. Biochem Biophys Res Comm 1993;191:399ŌĆō404.


16. Ballerman BJ, Zeidel ML, Gunning ME, Brenner BM. Vasoactive peptides and the kidney. The Kidney. 4th ed. In: Brenner BM, Rector FC, eds. Philadelphia: Saunders, 1991;510ŌĆō583.
17. Doria A, Warram JH, Krolewski AS. Genetic predisposition to diabetic nephropathy: evidence for a role of the angiotensin I-converting enzyme gene. Diabetes 1994;43:690ŌĆō695.


18. Tarnow L, Cambien F, Rossing P, Nielsen F, Hansen B, Lecerf L, Poirier O, Danilov S, Parving H. Lack of relationship between an insertion/deletion polymorphism in the angoitensin I-converting enzyme gene and diabetic nephropathy: and proliferative retinopathy in IDDM patients. Diabetes 1995;44:489ŌĆō494.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



