INTRODUCTION
Allergen challenge given to atopic patients results in early (immediate) and/or late reactions in target organs, including the skin,1, 2) nasal mucosa,3, 4) and bronchi.5, 6) Recently the potential importance ot IgE and mediators of mast cell and other inflammatory cells has been reported in the pathogenesis of late-phase allergic reactions.1, 2, 7ŌĆō9) The clinical relevance of late asthmatic reaction (LAR) has also been emphasized. Certain features of LAR resemble chronic, severe asthma, especially in that steroids are effective.10ŌĆō12) Possibly of most importance, LAR is followed by increased non-specific brochial reactivity.13ŌĆō16)
However, there is little evidence about what determines the occurrence of LAR. Robertson et al.6) noted that skin prick tests with ragweed extract elicited a wheal larger in diameter in subject with a dual asthmatic reaction (DAR) than in those with isloated early asthmatic reaction (EAR). Bouler et al.17) suggested that the occurrence of LAR could be predicted by the presence of a late cutaneous reaction (LCR) to the same antigen, but Price et al.18) failed to find a relationship between LCR and LAR. Maclntyre et al.19) noted that patients in whom the EAR was induced by a low dose of inhaled allergen were most likely to develop a LAR.
The purpose of the present study was to examine the patterns of early and late allergic reaction using extracts of the house dust mite, Dermatophagoides farinae, in the skin and in the bronchi. A practical objective was to find possible relationship between LCR and LAR to D. farinae.
MATERIALS AND METHODS
1. Subjects
Thirty adult perennial asthmatics, visiting the allergy clinic at Seoul National University Hospital, volunteered to participate in the study. All had a typical history of asthmatic symptoms, demonstrated a FEV1 variability of greater than 20%, and showed a positive ECR on the skin prick test with D. farinae extract. None of the subjects had received immunotherapy. At the time of the allergen challenge, the subjects, in each case, were free of sympotoms and without need of medications.
2. Allergen Extracts
For the skin prick test, the Bencard (U.K.) standard 1.2% D. farinae extract was used. For the intradermal skin test, 0.005% D. farinae extract was purchased from Torii/Hollister-Stier Co. in Japan. For the bronchial challenge, 1.2% carbol saline extract of D. farinae was provided by the Bencard Co. of the U.K.; it was freshly diluted for each test. The same batch of extracts was used throughout the study for both the skin prick and the bronchial provocation tests.
3. Skin Tests
The prick and intradermal tests were done at the same time without dilution of the extracts; the former was done on the back and the latter on the forearm of each subject. The ECR was read at 15 minutes, and interpreted as positive, if graded 2+ or more. We graded ECR ŽĢ, 1 +, 2 +, 3 +, 4 + in accordance with the recommended criteria of the Scandinavian Socierty of Allergology.20) The LCR was read 6 hours later, and graded I if the mean diameter of erythematous induration was less than 10 mm; II, if 10 to 20 mm; III, if 20 to 40 mm; and IV, if more than 40 mm. LCR graded III or more was interpreted as positive.
4. Bronchial Provocation Test
The control (saline) and the allergen were inhaled as aerosols generated by the Pari Inhalierboy nebulizer. Increasing concentrations of the allergen (1:1000 to 1:10 saline dilution of 1.2% D. farinae extract) were administered in five deep, consecutive inspirations at intervals of 20 minutes until a maximum concentration of the allergen was reached, or more than a 20% fall in FFV1 occurred. After completion of the challenge, FEV1 was measured at 10 minute intervals for 30 minutes, then at 30 minute intervals for 90 minutes, and thereafter, hourly for a total of 12 hours.
RESULTS
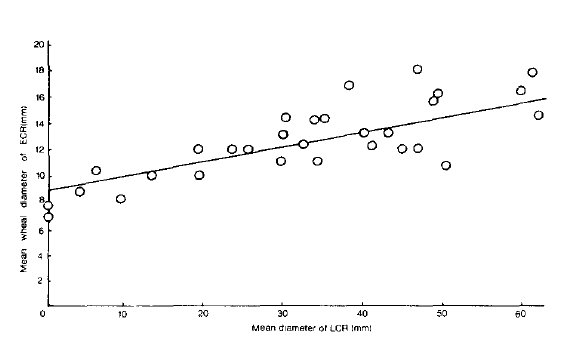
In the skin tests, LCRs were observed in nine subjects (33.3%) on the prick test, whereas they were observed in twenty-six (86.6%) on the intradermal test. There was a correlation between the size of the ECR and the size of the LCR on the intradermal test (r = 0.64, p<0.01) (Fig. 1). However, the size of the ECR had no relationship to the occurrence of the LCR on the prick test although there was no significant difference between the size of the ECR on the prick test and its size on the intradermal test. At the same time, patients whose ECR wheal sizes on the prick test were less than 5mm, elicited no LCR on the intradermal test.
In the bronchial provocation test, six patients showed no bronchoconstriction up to 12 hours after the allergen challenge, three showed isolated EAR, and twenty-one showed DAR. The LAR began 4 to 7 hours after the allergen inhalation and usually resolved within 24 hours. The concentration of D. farinae eliciting the EAR ranged from 2.4 ├Ś 10ŌłÆ5 to 1.2 ├Ś 10ŌłÆ3 w/v. Isolated EAR responders required a higher concentration (1.2 ├Ś 10ŌłÆ3 w/v) of D. farinae.
The relationship between skin and bronchial reactions is shown in Table 1 and 2. All patients with a negative LCR (grade II or less) on the intradermal test had no LAR to the bronchial challenge, whereas 21 of 24 subjects (87.5%) with a LCR showed a LAR. There was a tendency for an EAR to be provoked at a lower allergen concentration in patients with a large LCR (table 3).
All patients with a DAR had a specific serum IgE antibody to D. farinae, and 85.7% of them had increased total serum IgE levels (to over 300 u/ml) (Table 4). Other clinical factors such as patientsŌĆÖ age, duration of illness, or basal pulmonary funcation did not influence the occurrence a LAR.
DISCUSSION
In the use of either the prick or intradermal method of skin testing, an eary (immediate) wheal and flare reaction is often followed by a late-phase reaction. This phase may last 24 hours and the reaction is larger and generally more edematous than the early reaction. The late-phase reaction can be seen following challenges to the skin, nasal mucosa, and bronchi. They may be particularly important in the development of chronic asthma. When the late-phase skin reaction was originally described, it was though that the mechanism might have been a type 3 reaction (immune-complex-mediated) due to a precipitating IgG antiobody, as seen in bronchopulmonary aspergillosis.21)
However, precipitating antibodies have not been found associated with this late reaction, and further research has confirmed that the late reaction is an IgE-dependent sequel to the early reaction.1, 2, 22, 23)
Bronchial reaction to allergens, also, occurs in an early-and the late-phase. Sodium cromoglycate is a very effective agent used in the treatment of allergic asthma; it prevents both the early-and the late-phase reactions following bronchial provocation with an allergen.11,24 This implies that the development of a late reaction in the lung is dependent on an initial allergen/lgE/ mast cell interaction. Preventing degranulation with sodium cromoglycate prevents all subsequent events. If patients are pretreated with corticosteroids or prostaglandin sysnthetase inhibitors, late reactions alone are abolished leaving the early reactions unchanged.25ŌĆō27 This indicates a role for mast cell-derived arachidonic acid metabolites, such as prostaglandins and leukotriens in the late reaction.
Since there have been no established criteria for determining whether any given LCR should be considered positive we interpreted a LCR to be positive in this study when the mean diameter of the erythematous induration was over 20 mm. This decision was based on the observation that lesions smaller than this usually didnŌĆÖt show a gradual increase of reaction following ECR.
The occurrence of a LCR on the intradermal test was dependent on the size of the ECR. On the intradermal test, the LCR developed when preceded by an ECR, the wheal diameter of which was 10 mm or more (Fig. 1). This finding was almost identical with those of previous reports.1, 2, 9, 28, 29) Dolovich et al.1) found that an LCR was not seen unless the immediate wheal was about 8 mm or more, and Solley et al.2) found that a wheal of 15 to 20mm was required to induce the LCR. The elicitation rate of an LCR on a prick test was significantly lower than that an intradermal test (P<0.01) in this study, although there was no difference in the size of an ECR wheal on a prick test and on an intradermal test. This might be due to a difference in the ability of varying doses or strengths of allergen extracts to reach the tissue mast cells.
Boulet et al.17) who carried out titration to the end point in doing the skin test, noted that in patients with a DAR, a low antigenic concentration was required for a LCR, but they found no relationship between the size of a LCR and the pattern of airway response. We noted in this study that the larger the size of the LCR, the lower the allergen threshold for an EAR tended to be (Table 3). All the patients with a DAR had a DCR on the intradermal test. This relationship between cutaneous and bronchial reactions gave us the information that late skin and bronchial reactions might share common pathophysiologic mechanisms.
There have been a few reports about the existence of isolated LAR to the house dust mite.30ŌĆō33) Hills32) noted a high incidence of isloated LARs to the house dust mite in the patients less sensitive to it, and a conversion of an EAR to an isloated LAR when the concentration of inhaled allergen was reduced. However, this report is in conflict with that of Maclntyre et al.,19) who noted that EARs provoked by a low dose of D. pteronyssinus extract were most likely to be followed by LARs. In this study, the isolated LAR was not fully investigated, becuase all patients inhaled an increasing dose of allergen until an EAR was elicited or a maximum concentration of the allergen was reached. The frequency of the LAR among asthmatics varied in different reports from 33 to 73%. We observed an LAR, in 21 of 24 (87.5%) subjects who showed an EAR to D. farinae extract.
In this study, there was quite good agreement between late cutaneous reaction and late bronchial reaction to Dermatophagoides farinae in the patients with allergic asthma. It could be said that a late cutaneous reaction might be a simple clinical test for the prediction of a late bronchial reaction, although it should be expected that the various target organs would have a somewhat different reaction to the allergen.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





