INTRODUCTION
The sympathetic nervous system has an influence upon the secretion and the metabolism of the thyroid hormone, and conversely thyroid hormones can alter the responsiveness to endogenous catecholamines.1) In fact, the manifestations of hyperthyroidism, such as tachycardia, tremor, excessive sweating, nervousness, are those of the sympathetic hyperactivity and can be abolished with a beta-adrenergic antagonist, propranolol.2,3)
Since the production,4) turnover rate,5) and serum concentration6) of catecholamines are comparable to those of normal, an alteration of the beta-adrenergic receptor has been proposed as a possible molecular mechanism for the hyperadrenergic manifestations.7ŌĆō12)
Although many animal experiments have shown an increase of beta-adrenergic receptors in various tissues,11ŌĆō18 there has been no direct evidence about the alteration of beta-adrenergic receptors in the patients with hyperthyroidism until the first demonstration of beta-adrenergic receptors in peripheral lymphocytes by direct binding study.19) In that first study, Williams et al. reported no significant difference in beta-adrenergic receptors compared with normal controls. But there have been debates about the alteration of receptors. Ginsberg et al.20) demonstrated increased beta-adrenergic receptor density of mononuclear cells in T3-induced thyrotoxicosis. Anderson21) reported the increase of beta-adrenergic receptor number of leukocytes in hyperthyroidism. Furthermore, little has been known about the changes of beta-adrenergic receptors in hypothyroidism.
We therefore studied to observe the change of beta-adrenergic receptors with both human mononuclear and polymorphonuclear cells in various thyroid functional states. We also studied to test the hypothesis that alteration of beta-adrenergic receptors has tissue difference and to determine which cells are more useful in the receptor assay.
MATERIALS AND METHODS
Pharmacologic agents
[3H] dihydroalprenolol (DHA; S.A.; 92.4 Ci/mmol) was purchased from New England Nuclear Co. (┬▒) propranolol, dextran, ascorbic acid were obtained from Sigma Chemical Co.
Mononuclear and polymorphonuclear cell preparation
Between 0800ŌĆō0900 h, with the patients in a non-fasting state, 20ŌĆō30 ml blood were drawn from an antecubital vein into previously heparinized syringes. The separation procedure began within 30 min from the time of blood drawing at room temperature. Heparinized whole blood and 10 ml of 3% dextran in saline were mixed in a syringe and stood vertically for 30 min. And then the upper portion which included plasma and leucocytes were centrifuged on Ficoll-Hypaque density gradients, as described by Boyum,22) to separate mononuclear cells from polymorphonuclear cells. The isolated mononuclear cells were washed two times with HankŌĆÖs balanced salt solution. The polymorphonuclear cells contaminated with red blood cells were mixed with 6 ml of cold distilled water to lyze the red blood cells. 20 sec later, 2 ml of 3.5% NaCl was added and it was also washed with HBSS. Washed cells were resuspended in 50 mM Tris-HCl (pH 7.7), 10 mM MgCl2 (incubation buffer). Mononuclear cells contained 85% lymphocytes and 15% monocytes and polymorphonuclear cells were composed of 95% polymorphonuclear cells.
Both cells suspended in the buffer were destroyed for 30 sec by sonicator, and centrifuged at 1900 g for 10 min. The supernatant was recentrifuged at 41,000 g for 15 min at 4┬░C, and the pellet was suspended in incubation buffer to store at ŌłÆ 70┬░C until the binding assay. Protein was determined by the method of Lowry et al.23)
[3H] DHA binding assay
Binding experiments were conducted using the methods previously described by Davies et al.24) In these experiments, 100ŌĆō150 ╬╝g membrane protein were incubated with varing concentrations of [3H] DHA and 6 mM ascorbic acid for 20 min at 37┬░C. Specific binding was defined as the binding of [3H] DHA that was displaceable by 50 mM propranolol. Binding form was separated from the free form by glass fiber filter paper and counted in scintillation spectrophotometer. Binding assay was also performed at varing concentrations of membrane protein.
RESULTS
Binding of [3H] DHA to normal cells
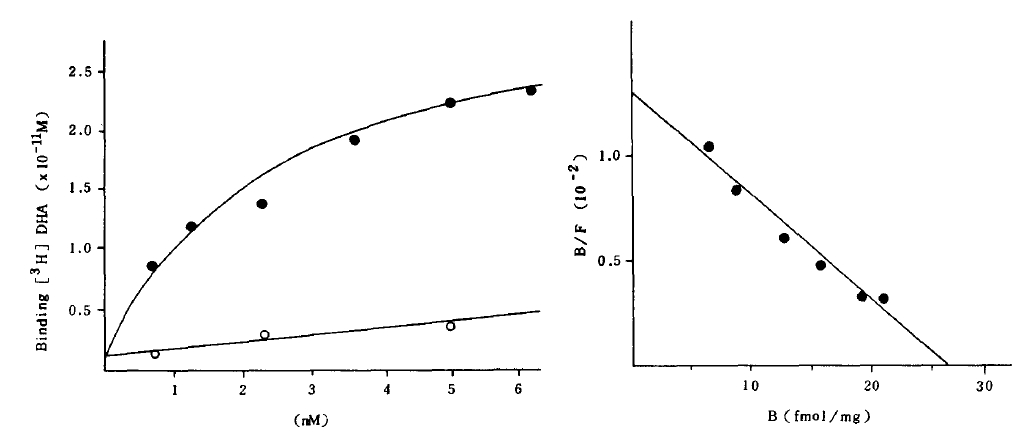
Total binding of [3H] DHA to both cells increased gradually to be saturated at the concentration of 6 mM [3H] DHA, while the nonspecific binding increased linearly.
A Scatchard analysis of the data gave a straight line which indicated beta-adrenergic receptor sites on both cells have the same affinity (Fig 1).
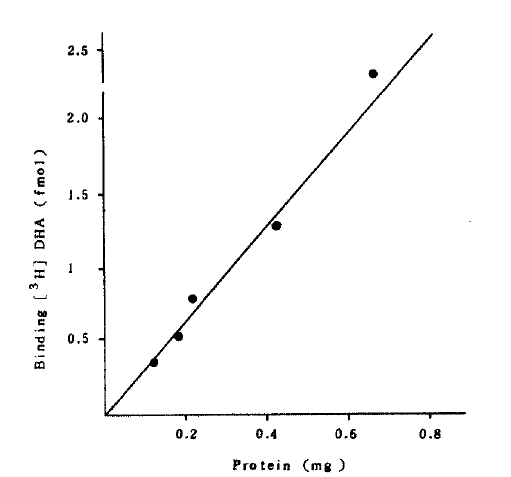
Specific binding of [3H] DHA was a function of membrance protein concentration (Fig 2).
Bindings of [3H] DHA to the patientŌĆÖs cells
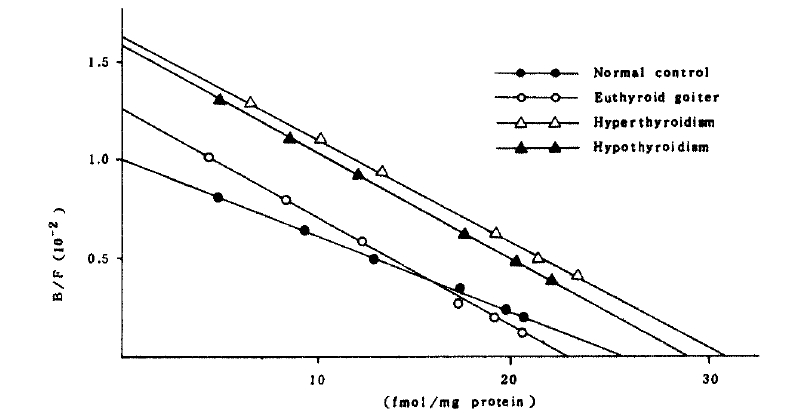
The affinity constant and concentration of beta-adrenergic receptors in both cells of the patients with euthyroid goiter were not significantly different from those of normal controls (Table 3 and Fig 3,4.).
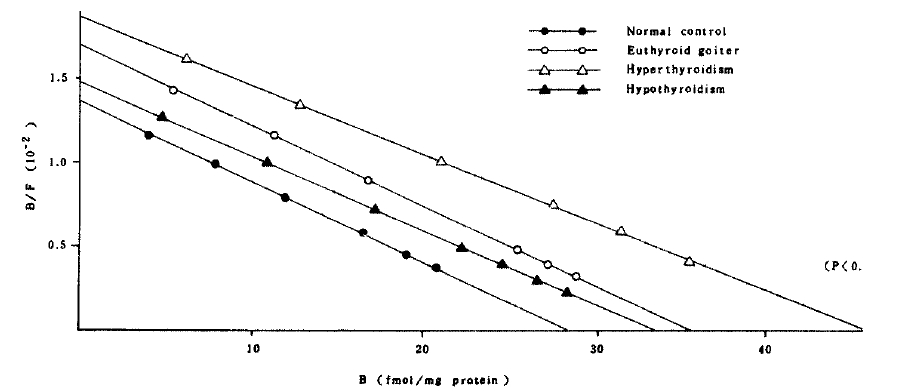
In the patients with hyperthyroidism, the mean concentration and the affinity constant were not significantly different from those of normal controls in mononuclear cell binding assay. But the mean concentration of the receptor in polymorphonuclear cells (46.07 ┬▒ 4.78 fmol/mg protein) significantly increased compared with that (28.42 ┬▒ 2.06 fmol/mg protein) of normal controls (Fig 4). It implies that the polymorphonuclear cells more sensitively represent the alteration of beta-adrenergic receptors. Interestingly, the patients whose receptor concentration of polymorphonuclear cells significantly increased also showed significantly high receptor concentration in mononuclear cells except only in two patients.
In the patients with hypothyroidism, the mean concentration and affinity constant of receptors were not significantly different from those of normal controls.
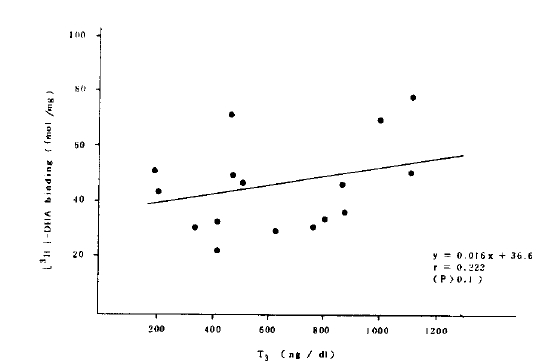
Although the receptor concentration of polymorphonuclear cells was significantly increased in the patients with hyperthyroidism, there was no correlation between the receptor concentration and thyroid hormone concentration (Fig.5).
DISCUSSION
Clinicians have thought that many of the symptoms of clinical hyperthyroidism are similar to what one would expect for a hyper-beta-adrenergic state. These include such manifestations as tachycardia, hyperdynamic circulation, increased pulse pressure, excessive sweating, anxiety, and tremor. This impression has been strengthened by the finding that propranolol, a beta-adrenergic antagonist, provides relief in many patients with symptomatic hyperthyroidism. Nonetheless, clear-cut evidence of increased sensitivity to beta-adrenergic catecholamines in patient with hyperthyroidism has been difficult to document. It seems clear that tissue and serum levels of catecholamines,6) sympathoadrenal catecholamine release,4) turnover rates of norepinephrine and of epinephrine5) are not elevated in patients with hyperthyroidism. Thus the increase in catecholamine receptor or postreceptor levels is more likely.
Many investigators have been interested in whether thyroid hormones might alter the concentration of beta-adrenergic receptors in tissues. The heart of a thyrotoxic rat showed highly statistically significant increase in the number of beta-adrenergic receptors without any change in affinity.7ŌĆō13) Futhermore, the number of beta-adrenergic receptors is decreased in the heart of hypothyroid rats8,12) and red blood cells of turkeys.25) But the application of direct binding studies using a biologically active radiolabeled compound to the investigation of possible molecular alterations of hormone receptors in human disease has been limited by the relative inaccessability of large quantities of human tissue for binding studies.
The presence of beta-adrenergic receptors in circulating leukocytes has been indicated by the demonstration in these cells of adenylate cyclase activity that responds to catecholamines with a typical beta-adrenergic specificity.26) Williams et al. first successfully identified beta-adrenergic receptors on the circulating mononuclear cells,19) and then they reported no significant difference in the binding of [3H] DHA between the patients with hyperthyroidism and euthyroid controls. But Ginsberg et al. demonstrated that triiodothyronine administration for one week resulted in objective evidence of moderate thyrotoxicosis and an increase in mean [3H] DHA binding which was attributable to an increase in beta-adrenergic receptor density.20) They proposed several possibilities that could explain the difference; The first possibility was that the effects of experimental thyrotoxicosis induced by the relatively short term administration of exogenous thyroid hormone are fundamentally different from those produced by relatively long term endogenous overproduction of thyroid hormones. Their subjects had no subjective manifestations of thyrotoxicosis and had serum triiodothyronine concentration lower than those of most spontaneously thyrotoxic patients. The second possibility was the biologic variation in mononuclear leukocytes beta-adrenergic receptor density. The third was the difference in [3H] DHA concentration used in the binding assays. They used the concentration of less than 5.0 nM compared with 10ŌĆō40 mM of Wiliams et al., which may result in some binding to low affinity sites that do not exhibit the characteristic of beta-adrenergic receptors.
Although some of our patients with hyperthyroidism showed a marked increment of receptor density in mononuclear cells, the mean receptor density was not significantly increased. It seems likely that this result was attributable to the heterogeneity of the patients or interindividual variation. The differences in the disease duration and degree of thyrotoxic manifestation may contribute to the heterogeneity of patients. The patients with recent onset less than three months tend to show an increased receptor density.
The mean receptor density of polymorphonuclear cells was significantly increased in the hyperthyroidism group. Nonetheless the marked variation was also observed in receptor density of polymorphonuclear cells, but the patients whose receptor density of mononuclear cell was not increased had a significant increase in polymorphonuclear cells. Polymorphonuclear cell seems to represent the alteration of beta-adrenergic receptor more sensitively. Polymorphonuclear cells have shorter life-span than mononuclear cells and c-AMP generation,27) enzyme secretion,28) phagocytosis, chemotaxis are more active and potent. Because polymorphonuclear cells seem to be more biologically active and so more prominently influenced by thyroid hormones, it is possible that those cells showed the alteration of beta-adrenergic receptor more sensitively in response to excessive thyroid hormones. In fact, polymorphonuclear cells have multiple physiologic functions which are sensitive in vitro to adrenergic modulation. Chemotaxis, phagocytosis, specific enzyme release in response to immunological stimuli are all decreased by beta-adrenergic agonist and this decrease is specifically prevented by beta-adrenergic antagonist.27,28)
Another possible explanation which is responsible for the lower sensitivity of mononuclear cells is the heterogenous cell population of mononuclear cells that is composed of lymphocytes and monocytes. But the ratio of T cell to B cell is less likely to be responsible for the alteration of receptor number. Bishoprice et al.29) found no difference in the beta-adrenergic receptor densities or affinities between T and B lymphocytes.
Andersson et al.21) reported that the number of beta-adrenergic receptor on polymorphonuclear cells was increased in the patients with untreated hyperthyroidism and returned to a normal level after treatment. Because the individual data was not shown in the report, it is obscure whether the number of beta-adrenergic receptor was increased in all patients or not.
The mechanism by which thyroid hormones increase the receptor number is not clear at this point. It is believed that thyroid hormones increase the synthesis of mRNA after its binding to the nucleoreceptor and result in the new synthesis of beta-adrenergic receptor.30)
There was no correlation between T3 concentration and receptor concentration in our study. It is obscure why all the patient did not show the increased number of receptor inspite of the high concentration of serum thyroid hormone and hyperadrenergic manifestation. But it suggests that the hyperadrenergic symptoms can not be explained completely only with the increase of receptor number. In fact, a few patient showed increase only in affinity constant.
Because an alteration in the pathway beyond hormone binding sites which also can be responsible for the changes of responsiveness to the adrenergic system,31) further investigation for the alteration of the postreceptor pathway is mandatory. The measurement of adenylate cyclase activity is currently under investigation. Our preliminary results disclosed that the enzyme activity increased in the patient with increased receptor number, but some patients showed the increased activity without alteration of the receptor. It is speculative that the disease duration mentioned above can influence the alteration of receptor and postreceptor pathways, resulting in sequential changes in postreceptor pathways after alteration of receptors.
Although the serum catecholamine concentration was increased in hypothyroidism,32) hypoadrenergic manifestations were frequently observed. Altered tissue responsiveness has been believed to be responsible, but the precise mechanism and location are not known. In animal experiments, the results about the alteration of receptor number were controversial. The receptor number of cardiac muscle cells and hepatocytes was increased, but that of adipocytes was not changed.33,34) Tse et al.9) observed that the generation of c-AMP in response to beta-adrenergic agonist was decreased although there was no change in the receptor number. Covalent modification of receptors seems to contribute to the decreased generation of c-AMP. Smith et al.35) demonstrated the number of beta-adrenergic receptors were not changed in human lymphocytes of the patients with hypothyroidism. Our results also showed no significant changes in the mean receptor number and affinity. But some patients had decreased number of receptors and some others had an increased number. And so the above mentioned disorders beyond receptors and the time-related changes of receptors should be observed in the future as in the case of hyperthyroidism.
In conclusion, the alteration of beta-adrenergic receptors seems to be responsible in part for the altered responsiveness to the adrenergic system. And the polymorphonuclear cell is thought to sensitively represent the alteration of receptor. But a disorder of other sites beyond the receptor should be investigated in the future.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





