 |
 |
| Korean J Intern Med > Volume 19(3); 2004 > Article |
|
Abstract
Background
Restenosis after percutaneous coronary intervention (PCI) is a matter that still remains to be resolved. Herein, the inhibitory effect of locally delivered 99mTcŌĆōHMPAO (hexamethyl propylene amine oxime) on neointimal hyperplasia after coronary stenting was examined in a pocine model, and its safety and efficacy observed in patients with coronary stent restenosis.
Methods
After a stent overdilation injury, local radioisotope delivery using 99mTcŌĆōHMPAO was applied to one coronary artery (Group I) and control therapy to another (Group II) in each of 10 pigs. Follow-up coronary angiogram (CAG) and histopathologic assessment were performed 4 weeks after stenting. Eleven patients (10 males and one female, 62.4┬▒5.7 years of age) underwent local administration of 30 mCi/2 mL 99mTcŌĆōHMPAO shortly after PCI, via a Dispatch CatheterŌäó, followed by a whole body scan to evaluate the distribution of the 99mTcŌĆōHMPAO, as well as a thalliumŌĆō201 (TIŌĆō201) myocardial scan to evaluate myocardial perfusion. The major adverse cardiac events (MACE) were assessed during a oneŌĆōyear clinical followŌĆōup.
Results
On histopathological analysis, the neointimal areas were 1.2┬▒0.6 and 2.7┬▒0.4 mm2 (p=0.002), and the histopathological areas of stenosis were 27.16.3 and 53.4┬▒5.2% in Groups I and II (p=0.001), respectively. In the clinical study, there was no inŌĆōhospital MACE. On a quantitative coronary angiographic analysis, the minimal luminal diameter was increased from 0.4┬▒0.3 to 2.9┬▒0.2 mm, and diameter stenosis decreased from 84.2┬▒9.5 to 16.3┬▒11.0% following PCI. Follow-up CAG was performed in 9 cases (81.8%) and restenosis occurred in 2 (22.2%). On a follow-up CAG, the minimal luminal diameter, diameter stenosis rate, lumen loss and loss index were 2.0┬▒0.8 mm, 27.7┬▒2.9%, 0.7┬▒0.7 mm and 0.2┬▒0.3, respectively. During the oneŌĆōyear clinical followŌĆōup there were no cases of death or acute MI, but two cases of target vessel revascularization (18.2%).
Coronary artery disease, such as angina pectoris and myocardial infarction, has sharply increased over the last 10 years, becoming a major reason of Korean adult death. Percutaneous transluminal coronary angioplasty (PTCA) was introduced to treat coronary artery disease, but caused restenosis in approximately 30ŌĆō40% of cases by negative remodeling of the injured artery and neointima formation1), and acute vessel closure, due to intimal dissection and thrombus formation, in approximately 10%2). Coronary artery stenting lessened acute vessel complications from 10ŌĆō15 to less than 1% after balloon dilation, establishing it as a universal PCI3). However, 20ŌĆō30% stent restenosis occurs due to neointima formation, a problem that still remains to be resolved4).
Various trials have recently been conducted on the prevention of stent restenosis following PCI, with local delivery of beta or gamma rays having proved effective5ŌĆō7). Herein, it is hypothesized that intracellular irradiation of the media and adventitia of the porcine coronary artery, by locally delivered 99mTechnetium hexamethyl propylene amine oxime (99mTcŌĆōHMPAO), could inhibit neointimal hyperplasia after a stent over dilation injury.
The local delivery of 99mTcŌĆōHMPAO has been shown to have a preventive effect on neointima hyperplasia and inŌĆōstent restenosis in a porcine coronary artery stent restenosis model. Therefore, whether the local delivery of 99mTcŌĆōHMPAO has a preventive effect on restenosis and is safety on humans were assessed.
Domestic pigs, 25ŌĆō35 kg in weight, were premedicated with 300 mg aspirin, 180 mg diltiazem, and 500 mg ticlopidine. Induction of general anesthesia was accomplished with intramuscular injections of ketamine (12 mg/kg) and xylazine (8 mg/kg). Local anesthesia of the mid-cervical region, using 2% lidocaine, was also applied prior to exposure and cut-down of the carotid artery using a midline cervical approach. The left carotid artery was cannulated with an 8 Fr. sheath. Under fluoroscopic guidance, with a CŌĆōarm (BVŌĆō25 Gold, Phillips, Best and Heerlen, Netherlands), catheters (7ŌĆō8 Fr.) were advanced to the coronary ostia. Throughout the duration of the invasive procedures, the pigs were supplied with continuous oxygenation via face masks. Saline was infused through the auricular vein. The electrocardiography and blood pressure were monitored continuously. This study was approved by the Ethical Research Committee of the Chonnam National University Hospital.
Twelve pigs received a local delivery of radiopharmaceuticals with either 99mTcŌĆōpertechnetate (n=5) or 99mTcŌĆōHMPAO (n=7). Upon positioning of the catheter at the target region of the coronary artery, 1,110 MBq (555 MBq/mL) of the radiopharmaceutical were infused, at a rate of 1 mL/min, followed by infusion of physiologic saline for 5 minutes. The actual radiation dose delivered was determined using a dose calibrator on dissected coronary arteries. The residual radiation in the syringe and catheters were quantified and subtracted from the initial administered dose.
Experiments were performed on ten pigs. In the control group (n=3), balloons were attached to PalmazŌĆōSchatz stents (Johnson & Johnson, Piscataway, NJ) and inflated in the coronary artery to 1.3 times the reference vessel diameter. In the experimental group (n=7), 1,110 MBq (555 MBq/mL) 99mTcŌĆōHMPAO was delivered via a Dispatch Catheter (Boston Scientific, Boston, MA) prior to balloon dilation, as described for the control group. 99mTcŌĆōHMPAO was infused using an infusion pump, such that a 1,110 MBq dose would occupy 2 mL of the syringe, at a rate of 1 ml/min followed by infusion of physiologic saline for 5 minutes. The diameter of the Dispatch catheter and balloon selection (3ŌĆō4 mm) was determined by quantitative analysis of the coronary angiograms. Spiral balloons on the Dispatch Catheter were deployed with 4 atm of pressure. During local delivery of the 99mTcŌĆōHMPAO, a radiocontrast dye was infused through the central channel to demonstrate the blood flow distal to the Dispatch catheter. Precautions were taken to avoid ischemia in the distal segment during the 99mTcŌĆōHMPAO delivery. A PalmazŌĆōSchatz stent was placed in the right coronary artery (RCA) and expanded with 8 atm of pressure for 20 sec using a nonŌĆōcompliant PTCA balloon catheter and standard indeflater. Neither heparin nor nitrates were administered after placement of the stent. Upon completion of the experiments, the carotid artery was repaired, and the incision site was sutured. FollowŌĆōup angiograms were obtained four weeks after the experiments. Quantitative analysis was performed with an image analyzer (Cardio 500, Kontron Inc., Eden Prairie, MN).
One pig was sacrificed after the local 99mTcŌĆōHMPAO delivery. The hearts was extracted, rinsed, and the epicardial coronary arteries identified. A large section, including the entire coronary artery and surrounding myocardium, was obtained. Sections were placed in a container with dry ice vapor and packed in dry ice. Using a microtome, 20ŌĆō40 mm sections were made, mounted on glass slides, and air-dried. In a darkroom, the glass slides were covered with X-ray film (NMB film) and stored at 70┬░C. The exposure times were 2, 8 and 18 hours. After developing the autoradiographs, the glass slides were stained with Hematoxylin and Eosin (H&E) 8, 9). Densitometry was performed using Image Pro Plus (Media Cybernetics, Silver Spring, MD).
The amount of radiation absorption in the vascular wall was determined according to the Monte Carlo Simulation Study using the EGS4 code. A cylindrical model was implemented in the description of the vessel. Assuming a 1 mm central lumen, the intima, media and adventitia were described as concentric cylinders, with wall thickness of 0.27, 0.82 and 0.12 mm, respectively. The distribution of 99mTcŌĆōHMPAO was determined by setting the target volume to the smooth muscle layer. A computer analysis was repeated until the standard deviation (SD) was less than or equal to 5%.
Thierens et al.10) reported that intracellular radiation in lymphocytes, by Auger electrons from 99mTcŌĆōHMPAO, was equivalent to 20 to 30 times the external x-ray irradiation. Thus, the theoretical dosimetry was calculated as the number of cells in the coronary arterial wall exposed to intracellular irradiation from 99mTcŌĆōHMPAO.
After follow-up coronary angiography, the pigs were sacrificed by an intravenous injection of barbiturates or KCI. The extracted porcine heart was fixed in 10% formalin solution for 24 hr. The stents in the isolated coronary arteries were easily identified under fluoroscopic guidance and were also readily palpable. Each coronary artery was dissected to remove the stented portion, including a 1 cm vessel segment both proximal and distal to the stent. Sections of the stented portion were taken at 2ŌĆō3 mm intervals with a stereomicroscope to avoid distortion of or damage to the artery. Each section of the stented portion of the coronary artery was H&E stained. In order to assess neointimal proliferation, immunohistochemistry was performed using a murine monoclonal antibody (clone PC 10, Dako, Carpinteria, CA) to the proliferating cell nuclear antigen (PCNA). All morphometric analyses were performed using image analysis systems, according to previously established methods11, 12). The lumen diameters were determined using calibrated digital microscopic planimetry. Subtraction of the crossŌĆōsectional areas of the lumen (luminal area), internal elastic lamina (IEL) and external elastic lamina (EEL) from that of the vessel wall yielded the crossŌĆōsectional area of the neointima and media. Reference values for the vessel wall thickness were obtained from the averages of those found 1 cm proximal and distal to the stented region. The neointimal crossŌĆōsectional area was determined by subtracting the luminal area from that demarcated by the IEL. The degree of restenosis was calculated as the percent area of restenosis (%) = 100 (1ŌĆōluminal area/IEL area).
Of the patients attending the heart center the Chonnam National University Hospital between June and Oct. 2001, 11 (10 males, mean age 62.4┬▒5.7 years) that had had a coronary artery stent restenosis lesion and simultaneous local delivery of 99mTcŌĆōHMPAO the same way as in the animal experiment following PCI, were entered onto this study
The subject patients were administered 1.100MBq (30 mCi)/2 mL, with the Dispatch CatheterŌäó following a successful PCI, in the same way as in the animal experiments. Both preŌĆō and postŌĆōPCI, electrocardiogram, CBC, and chemistry tests were performed.
From quantitative coronary angiography, some patients were chosen whose vessel diameter and lesion length were 2.5ŌĆō4.0 and under 20 mm, respectively. The informed consent of these patients were obtained. Patents with acute myocardial infarctionwithin 72 hours, a thrombusŌĆōcontaining target lesion, graft vessel PCI and chronic total occlusion were excluded, and those with more than 30% remaining stenosis postŌĆōPCI and no complications, such as coronary artery dissection, acute vessel closure or myocardial infarction, were included.
Coronary angiography was performed before and 6 months after PCI. Significant coronary artery stenosis was defined as over 50% stenosis of the major coronary artery or of the diameter of the major branch. The morphological classification of the stenotic areawas in accordance with the guidelines of the American College of Cardiology/American Heart Association (ACC/AHA). The reference vessel and minimal luminal diameters were measured using the Philips H5000 or Allular DCI program pre and postŌĆōPCI. Every patients took aspirin (100ŌĆō200 mg) and either ticlopidine (500 mg) or clopidogrel (75 mg) one or twice a day preŌĆōPCI, and aspirin constantly and ticlopidine or clopidogrel for 6 months postŌĆōPCI. PCI was undertaken on the coronary artery lesion using the currently preferred techniques. The femoral artery sheath was removed 6 hours postŌĆōPCI, and heparin was only administered if there was complication. Compression of the vascular access site was performed for 20 minutes by a wellŌĆōtrained doctor, and then pressed employing a Femostop device. Following PCI, those cases where the remaining restenosis was under 30%, myocardial infarction was not observed on EKG, cardiac enzyme assay, and major complications, such as emergent coronary artery bypass graft (CABG), and coronary revascularization, or death did not doccur, were considered successful. InŌĆōstent restenosis was classified by the criteria of Mehran et al13), which was deemed to have occurred when the restenosis was over 40% that of the normal diameter.
As the follow-up the major adverse cardiac events (MACE) and survival were analyzed once a month at the out patients department, InŌĆōhospital MACE were defined as cardiac death, myocardial infarction, CABG, any stroke, and target lesion revascularization. The primary end point was the result of quantitative coronary angiography from the followŌĆōup coronary angiography by 6 months; the second end point was cardiac death, myocardial infarction, target vessel revascularization or a stroke.
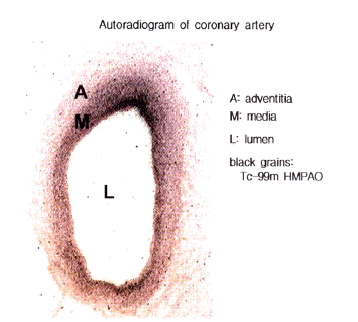
The delivery of 99mTcŌĆōHMPAO into the coronary arterial wall was 3.17┬▒0.67%, while that of 99mTcŌĆōpertechnetate was 0.01┬▒0.01% (Table 1). Numerous small grains were shown to be distributed in coronary arterial wall after 18 hour exposure in autoradiogram. No grains appeared in the autoradiograph of the control sections. The coronary artery and surrounding myocardium had clearly appreciated. The relative radioactivities of each layer of the vessel wall were 7.6, 59.7, 11.2 and 21.5% in the intima, media, adventitia and surrounding myocardium, respectively. The radioactive particles in the vessel wall were unevenly distributed in the intima area: media area: adventitia area was 1:7:2 (Figure 1).
The dosimetry in the smooth muscle layer of the porcine coronary arteries, as determined by the Monte Carlo Simulation Study, was 0.67┬▒0.14 Gy (range, 0.45ŌĆō0.94 Gy). Assuming the diameter of the porcine coronary artery to be 1 mm, the length of the Dispatch catheter micropores 2 cm, the thicknesses of the media and adventitia 0.94 ╬╝m and the diameter of a cell to be 10 ╬╝m, then the number of cells exposed to 99mTcŌĆōHMPAO would be about 1 ├Ś 108. The dose of 99mTcŌĆōHMPAO delivered to the porcine coronary arterial wall by the Dispatch Catheter in this study was 35.19┬▒7.44 MBq, which was 3.17┬▒0.67% of 1,110 MBq. Therefore, the exposed dose to the cells in the coronary arterial wall, according to Thierens et al10), was equivalent to 20 to 30 Gy of external XŌĆōray irradiation10).
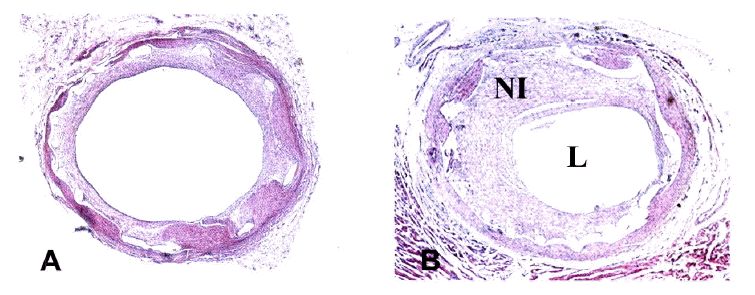
On quantitative coronary angiographic analysis, the percentage diameter of stenosis in the group receiving 99mTcŌĆōHMPAO was significantly lower than that seen in the controls (7.28┬▒5.50% in group I and 16.43┬▒3.70% in group II, p<0.05) (Table 2). On histopathological analysis, there was no difference in the media area between the two groups; 1.4┬▒0.5 and 1.1┬▒0.3 mm2 in groups I and II, respectively. The neointima areas were 1.2┬▒0.6, and 2.7┬▒0.4 mm2, the histopathological stenosisareas were 27.1┬▒6.3 and 53.5┬▒5.2% in groups I and II, respectively, and thus the group that had undergone radiotherapy had remarkably ssmaller neointima and histopathological stenosis areas than the other group (each p=0.002, p=0.001) (Table 3, Figure 2).
In 10 male patients (90.0%) in this clinical study, there were 1 and 8 with stable and unstable angina pectoris, respectively, and of those with old MI there were 2, 7, 7, 3 and 1 with cardiac risk factors, hypertension, smoking, diabetes mellitus, and hypercholesterolemia. The ejection fraction was 63.2┬▒5.8% (Table 4).
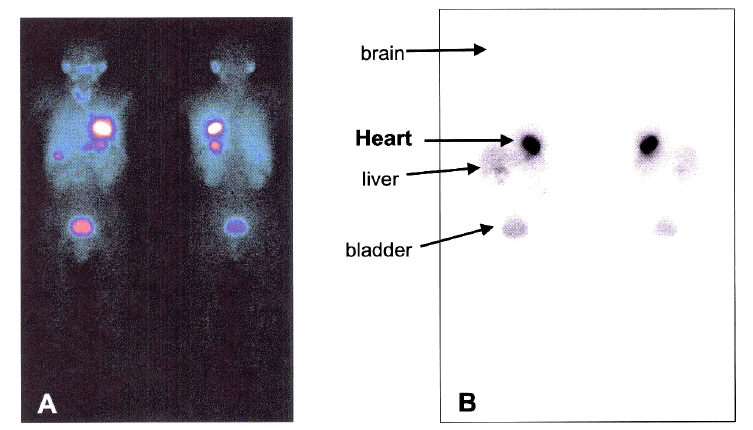
On a whole body and heart SPECT (Figure 3), 27.5┬▒5.67% of the delivery dose was observed in the heart area.
The locations of the lesion vessels were the left anterior descending artery, 4 the right coronary artery and 3 the left circumflex artery in 4, 4 and 3, respectively; the types of lesion were the B1, B2 and types, by the ACC/AHA classification, in 3, 4 and 4, respectively. Types of stent restenosis were I, II, III, IV in 3, 6, 1 and 1, respectively; with lesion lengths of restenosis under 10, and 10ŌĆō20 mm in 2 and 9, respectively. A balloon angioplasty was carried out in 10 patients and a cutting balloon angioplasty in a further 1. A quantitative coronary angiographic analysis showed better results compared to preŌĆōPCI to postŌĆōPCI that minimal luminal diameter was from 0.04┬▒0.31 mm to 2.89┬▒0.29 mm, diameter stenosis rate was from 84.20┬▒9.52% to 16.35┬▒11.06% and acute luminal gain was 2.57┬▒0.38. On the follow-up coronary angiography, the minimal luminal diameter, diameter stenosis rate, lumen loss and loss index were 2.08┬▒0.83 mm, 27.7┬▒9.1%, 0.79┬▒0.78 mm and 0.23┬▒0.30, respectively (Table 5, 6).
There were no major inŌĆōhospital cardiac complications. All the patients were followed up for 1 year. A followŌĆōup coronary angiography was performed on 9 patients (81.8%) and 2 of them had restenosis (22.2%). During the 1 year clinical follow-up, there wereas no cardiac deaths, acute myocardial infarctions, strokes or CABG, but 2 (18.2%) target vessel revascularizations (Table 7).
No patients had neither sideŌĆōeffects nor complications related to the PCI, and all the results from laboratory examinations performed pre and postŌĆōPCI and in after 6th month were within normal ranges, with no considerable differences (Table 8).
Stent restenosis due to neointima hyperplasia is considered a major difficulty of PCI. The exact reason for the neointima hyperplasia was not clear, but was known to be related to the proliferation of the drelocated smooth muscle cells from the vessel media layers. Various treatments; gene therapy, radiotherapy, drug-eluting or coated stents, are being trialed for the prevention of neointima hyperplasia, with local radiation delivery to the coronary artery being study as one of the effective treatments5ŌĆō7, 14).
Therefore local radiation delivery could prevent proliferation of the neointima. Beta and gamma rays, as radioisotopes for local radiation delivery, are being studied. GammaŌĆōrays had good results in a study of 192IrŌĆōtreated patients with stent restenosis15). BetaŌĆōrays were used, by Verin et al, for the study of local radiation delivery16), which showed the 1st indication of radiotherapy in the coronary artery iwas an inŌĆōstent restenosis lesion.
166Holmium(166HoŌĆōcoated balloon) was developed for us, with the support of the Korea Atomic Energy Research Institute, for inŌĆōstent restenosis lesions which improved the function of the coronary artery endothelial cells and suppressing neointima formation within the stent17, 18) in a porcine coronary artery stent restenosis model. The local delivery of 99mTcŌĆōHMPAO has also been reported to have an effect in preventing stent restenosis and controlling inŌĆōstent neointima hyperplasia in a porcine coronary stent restenosis model19).
99mTcŌĆōHMPAO is a lipophilic agent that can pass through the bloodŌĆōbrain barrier as well as diffuse through cell membranes. Once internalized in the intracellular compartment, 99mTcŌĆōHMPAO is converted to a waterŌĆōsoluble form, which for the most part becomes stored in cells. An understanding of theis nature of 99mTcŌĆōHMPAO has led to the development of applications in the imaging of cerebral blood flow and the detection of infection by the labeling of leukocytes20ŌĆō22). From the labeling of heparin with 99mTc, Camenzind et al.23) reported that 2.5┬▒2.4% of the heparin could be locally delivered into the human coronary arterial wall by a Dispatch Catheter. The present study revealed that 2ŌĆō5% (mean 3.17%) of the heparin can be delivered locally, which was similar to the results of Camenzind et al23). The delivered 99mTcŌĆōHMPAO was believed to be localized inside the arterial cells due to the lipophilicity of 99mTcŌĆōHMPAO.
Little data is available concerning the irradiation dosimetry of intracellular 99mTcŌĆōHMPAO, other than for lymphocytes. Accurate calculation of the dose is very difficult because of the different Auger electron groups emitted in the decay of 99mTc, the distribution of the radioactivity within the cells and the limits of the classical dosimetry methods. Thierens et al.10) reported that the radiation damage in 1.3 ├Ś 108 lymphocytes, due to self-irradiation with 740 MBq of 99mTcŌĆōHMPAO, was estimated to be equivalent to that caused by 26 Gy of XŌĆōrays. They estimated the dose using a biological dosimetry method of a micronucleus assay, with extrapolation of the data. In their experiment the total radioactivity in 107 lymphocytes was 28 MBq after a 740 MBq dose, and this dose almost completely inhibited the ability of the lymphocytes to proliferate. A few other studies have reiterated that 99mTcŌĆōHMPAO could deliver doses of radiation equivalent to those provided by high dose external radiation24ŌĆō26). The calculated absorbed dose to the cells in a labeling procedure for 108 granulocytes with 500 MBq 99mTc, with a bisalt method without pretinning, yielded a value of 17.7 Gy, assuming an uniform distribution of intracellular activity24).
Herein, about 1 ├Ś 108 cells were assumed to be irradiated from 37 MBq of 99mTcŌĆōHMPAO, which was located intracellularly. According to the report of Thierens et al., the exposure dose from intracellular 99mTcŌĆōHMPAO was equivalent to 20 to 30 Gy of external XŌĆōray irradiation10). In suppressing the proliferation of damaged vessel walls, 8ŌĆō30 Gy was found to be effective27, 28). So the dose of intracellular irradiation from 99mTcŌĆōHMPAO seems to be adequate to inhibit the proliferation of medial and adventitial cells. Autoradiography confirms that the 99mTcŌĆōHMPAO is mainly retained in the media and adventitia of coronary arterial wall. The calculation for the dosimetry in the coronary arterial wall, according to the Monte Carlo simulation study, showed only 0.67┬▒0.14 Gy, which was not relevant to the result of this study, where the neointimal proliferation was significantly inhibited. The rate of 99mTcŌĆōHMPAO absorption was estimated from the injected amount vs. amount found in the heart on the whole body and heart SPECT of the patients. On the SPECT, 27.5┬▒5.67% of injected amount was observed in the heart cells.
The best merit of 99mTcŌĆōHMPAO on local delivery is its ease of application. That is to say, even in hospital with no atomic facility, it would be possible to produce material in the form of a commercial kit within 1 hour, which would be capable of being used for radiotherapy, as long as there is a doctor able to control the radioisotopes. It is also economical, as it costs less than other radiotherapies.
In this study, there were no MACE, with about 20% target vessel revascularization, compared to the 16ŌĆō31% in GAMMA I6), START29) and INHIBIT trials30), and gave a good result. Even if though this was a small trial, the patientsŌĆÖ lesions were relatively longer than 10mm, and considering 8 of our patients that did not have focal, but diffuse inŌĆōstent restenosis lesions, our result can be regarded as good. All patients in this study had no conventional complications of radiotherapy, such as late stent thrombosis or edge failure, which was probably due to the few patients.
The first limitation of this study was that, there were only a few patients. However, an advanced study will be required as a trial of this novel radiotherapy. The second limitation was the accuracy of the dosimetry of irradiation to the tissue may have been lower than that estimated. The determination of the radiation doses, according to the amount absorbed by the whole body and heart, may cause low accuracy as the delivery catheter thickness, lesion vessel size and irradiating time were not taken into consideration. The third limitation related to the Dispatch CatheterŌäó used for the local delivery, which could be a cause for practical concern.
In conclusion, the local administration of 1,110 MBq 99mTcŌĆōHMPAO into the coronary arterial wall, using a Dispatch Catheter, delivered 35.1┬▒7.44 MBq, which was 3.1┬▒0.67% of the injected dose, mainly into the media and adventitia of the coronary arterial wall. This novel local radiotherapy with 99mTcŌĆōHMPAO is feasible to cure inŌĆōstent restenosis lesions in animals and clinical experiments. However, further clinical studies might be required in the future.
Notes
This research was supported by grants from the Korean Ministry of Health and Welfare, HMP-98-M-5-0059
Figure┬Ā1.
Autoradiography of a porcine coronary artery after an 18-hour exposure. Grains of 99mTc-HMPAO were distributed mainly in the intima and media of the coronary artery. The relative radioactivities of each layer of the vessel wall were 7.6, 59.7, 11.2 and 21.5% in the intima, media, adventitia and the surrounding myocardium, respectively.
Figure┬Ā2.
Histopathological findings of the local delivery of 99mTc-HMPAO (A) and the control (B) porcine coronary arteries. A higher volume of neointima and degree of area stenosis was observed in the control than the irradiated artery. NI, neointima; L, lumen.
Figure┬Ā3.
Whole body scan finding after the local delivery of 99mTc-HMPAO. The distribution rate was calculated as 100├Ś (count of target organ/count of whole body), and in this case was 26.7% 3 hours after the intracoronary local delivery of 99mTc-HMPAO. A higher uptake by the heart was noted in the whole body scan.
Table┬Ā1.
Local delivery efficacy of radiopharmaceuticals into the porcine coronary artery
Table┬Ā2.
Quantitative coronary angiographic findings in irradiated porcine coronary arteries (Group I) and control arteries (Group II)
| Group I | Group II | |||
|---|---|---|---|---|
|
|
||||
| baseline | After 4weeks | baseline | After 4weeks | |
| PRD (mm) | 2.90┬▒0.2 | 2.93┬▒0.8 | 2.81┬▒0.2 | 2.79┬▒0.1 |
| DRD (mm) | 2.60┬▒0.3 | 2.73┬▒0.9 | 2.13┬▒0.2 | 2.25┬▒0.4 |
| RD (mm) | 2.75┬▒0.2 | 2.83┬▒0.8 | 2.60┬▒0.0 | 2.53┬▒0.2 |
| DS (%) | 7.28┬▒5.5 | 16.43┬▒3.7* | ||
Table┬Ā3.
Histopathological assessment of irradiated porcine coronary arteries (Group I) and control arteries (Group II)
| Group I | Group II | p | |
|---|---|---|---|
| Neointima area (mm2) | 1.25+0.60 | 2.76┬▒0.40 | 0.002 |
| Media area (mm2) | 1.43┬▒0.50 | 1.11┬▒0.30 | 0.336 |
| Area stenosis (%) | 27.1┬▒6.3 | 53.5┬▒5.2 | 0.0001 |
Table┬Ā4.
Baseline clinical characteristics of the patients
Table┬Ā5.
Coronary angiographic characteristics
Table┬Ā6.
Quantitative coronary angiographic results
Table┬Ā7.
One-year clinical follow-up
Table┬Ā8.
Laboratory findings before, and follow-up after, percutaneous coronary intervention
REFERENCES
2. Lincoff AM, Popma JJ, Ellis SG, Hacker JA, Topol EJ. Abrupt vessel closure complicating coronary angioplasty: clinical angiographic and therapeutic profile. J Am Coll Cardiol 19:926ŌĆō9351992.


3. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P. A comparison of balloonŌĆōexpandable stent implantation with balloon angioplasty in patients with coronary heart disease. N Eng J Med 331:489ŌĆō4951994.

4. Fischman DL, Leon MB, Bairn DS, Schatz RA, Savage MP, Penn I, Detre K, Vettri L, Ricci D, Nobuyoshi M. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 331:496ŌĆō5011994.


5. Waksman R, White RL, Chan RC, Bass BG, Geirlach L, Mintz GS, Satler LF, Mehran R, Serruys PW, Lansky AJ, Fitzgerald P, Bhargava B, Kent KM, Pichard AD, Leon MB. Intracoronary gammaŌĆōradiation therapy after angioplasty inhibits recurrence in patients with inŌĆōstent restenosis. Circulation 101:2165ŌĆō21712000.


6. Leon MB, Teirstein PS, Moses JW, Tripuraneni P, Lansky AJ, Jani S, Wong SC, Fish D, Ellis S, Holmes DR, Kerieakes D, Kuntz RE. Localized intracoronary gammaŌĆōradiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med 344:250ŌĆō2562001.


7. Waksman R, Bhargava B, White L, Chan RC, Mehran R, Lansky AJ, Mintz GS, Satler LF, Pichard AD, Leon MB, Kent KK. Intracoronary betaŌĆōradiation therapy inhibits recurrence of inŌĆōstent restenosis. Circulation 101:1895ŌĆō18982000.


8. Tomoike H, Ogata I, Maruoka Y, Sakai K, Kurozumi T, Nakamura M. Differential registration of two types of radionuclides on macroautoradiograms for studying coronary circulation: concise communication. J Nucl Med 24:693ŌĆō6991983.

9. Greiff J. Bone healing in rabbits after compression osteosynthesis, studied by TcŌĆō99m(Sn)polyphosphate scintimetry and autoradiography. J Nucl Med 22:693ŌĆō6981981.

10. Thierens HM, Vral AM, van Haelst JP, van de Wiele C, Schelstraete KH, de Ridder LI. Lymphocyte labeling with technetiumŌĆō99mŌĆōHMPAO: a radiotoxicity study using the micronucieus assay. J Nucl Med 33:1167ŌĆō11741992.

11. Schneider JE, Berk BC, Gravanins MB, Santoian EC, Cipolla GD, Tarazona N, Lassegue B, King SB 3rd. Probucol decreases neointimal formation in a swine model of coronary balloon injury. Circulation 88:628ŌĆō6371993.


12. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Park CS, Jung SW, Cho JG, Park JC, Kang JC. The preventive effects of heparinŌĆōcoated stent on restenosis in the porcine model. Catheter Cardiovasc Interv 48:324ŌĆō3301999.


13. Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of inŌĆōstent restenosis: classification and implications for longŌĆōterm outcome. Circulation 100:1872ŌĆō18781999.


14. Jeong MH, Ahn YK, Cho JG, Park JC, Kang JC. Successful coronary stent implantation using local NO donor delivery. J Interv Cardiol 13:191ŌĆō1952000.

15. Teirstein PS, Massullo V, Jani S, Russo RJ, Cloutier DA, Schatz RA, Guarneri EM, Steuterman S, Sirkin K, Norman S, Tripuraneni P. TwoŌĆōyear followŌĆōup after catheterŌĆōbased radiotherapy to inhibit coronary restenosis. Circulation 99:192ŌĆō1941999.


16. Verin V, Popowski Y, de Bruyne B, Baumgart D, Sauerwein W, Lins M, Kovacs G, Thomas M, Caiman F, Disco C, Serruys PW, Wijns W. Endoluminal betaŌĆōradiation therapy for the prevention of coronary restenosis after balloon angioplasty. N Engl J Med 344:243ŌĆō2492001.


17. Rhew JY, Jeong MH, Lee SR, Hong YJ, Lee SH, Park OY, Jeong WK, Kim W, Kim JH, Yum JH, Song HC, Bom HS, Park KB, Ahn YK, Cho JG, Park JC, Baik YH, Kang JC. The effects of radiation using HoŌĆō166 on endothelial function in a porcine coronary model. Korean Circ J 32:118ŌĆō1242002.

18. Kim W, Jeong MH, Park OY, Rhew JY, Bom HS, Choi SJ, Park KB, Kim EH, Kim JH, Ahn YK, Park JT, Cho JG, Park JC, Kang JC. Effects of betaŌĆōradiation using holmiumŌĆō166 coated balloon on neointimal hyperplasia in a porcine coronary stent restenosis model. Circ J 67:625ŌĆō6292003.


19. Jeong HJ, Bom HS, Song HC, Ahn YK, Kim NH, Cho JH, Kim EH, Jeong MH, Kang JC. Inhibition of coronary stent restenosis by local delivery of TcŌĆō99m HMPAO. J Nucl Med 41:797S. 2000.
20. Huang WT, Lo JM, Kao CH, Wang SJ. 99mTc phenyiene imine phenol as a potential leukocyte labeling agent. Nucl Med Commun 18:66ŌĆō691997.


21. Asenbaum S, Brucke T, Pirker W, Pietrzyk U, Podreka I. Imaging of cerebral blood flow with technetiumŌĆō99mŌĆōHMPAO and technetiumŌĆō99mŌĆōECD: a comparison. J Nucl Med 39:613ŌĆō6181998.

22. Borch K, Greisen G. 99mTcŌĆōHMPAO as a tracer of cerebral blood flow in newborn infants. J Cereb Blood Flow Metab 17:448ŌĆō4541997.


23. Camenzind E, Bakker WH, Reijs A, van Geijlswijk IM, Boersma E, Kutyk MJ, Krenning EP, Roelandt JR, Serruys PW. SiteŌĆōspecific intracoronary heparin delivery in humans after balloon angioplasty: a radioisotopic assessment of regional pharmacokinetics. Circulation 96:154ŌĆō1651997.


24. Skretting A, Benestad HB, Sundrehagen E. Whole body distribution of 99mTc labelled autologous human granulocytes and radiation dose to cells and organs. Eur J Nucl Med 14:1ŌĆō71988.


25. Makrigiorgos GM, Adelstein SJ, Kassis AI. Limitations of conventional internal dosimetry at the cellular level. J Nucl Med 30:1856ŌĆō18641989.

26. Merz T, Tatum J, Hirsch J. TechnetiumŌĆō99mŌĆōlabeled lymphocytes: a radiotoxicity study. J Nucl Med 27:105ŌĆō1101986.

27. Teristein P. ╬▓ŌĆōradiation to reduce restenosis: too little, too soon? Circulation 95:1095ŌĆō10971997.


28. Waksman R, Rodriguez JC, Robinson KA, Cipolla GD, Crocker IR, Scott NA, King SB 3rd, Wilcox JN. Effect of intravascular irradiation on cell proliferation, apoptosis, and vascular remodeling after balloon overstretch injury of porcine coronary arteries. Circulation 96:1944ŌĆō19521997.


29. Popma JJ, Suntharalingam M, Lansky AJ, Heuser RR, Speiser B, Teirstein PS, Massullo V, Bass T, Henderson R, Silber S, von Rottkay P, Bonan R, Ho KK, Osattin A, Kuntz RE. A randamised trial of 90strontium/90yttrium beta radiation versus placebo control for the treatment of inŌĆōstent restenosis. Circulation 106:1090ŌĆō10962002.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



