 |
 |
| Korean J Intern Med > Volume 19(1); 2004 > Article |
|
Abstract
Cryptococcus albidus, a non-neoformans species of the genus Cryptococcus, is generally regarded as a rare cause of disease. There have been only 14 previously reported cases in which this organism has been isolated as a pathogen, none of which occurred in a renal transplant recipient. A 23-year-old renal transplant recipient taking medication consisting of cyclosporine and prednisolone was admitted with a 10-day history of dry cough, fever and progressive dyspnea. The next day, his respiratory status deteriorated dramatically, and he developed acute respiratory distress syndrome (ARDS) and fulminant septic shock. On the eighth hospital day, tender macules on both his shins coalesced to form erythematous patches. Cryptococcus albidus was isolated by skin biopsy and tissue culture. We report here the first case of disseminated cryptococcosis caused by C. albidus in a renal transplant recipient who had been successfully treated with fluconazole monotherapy.
Cryptococcosis is a serious opportunistic fungal infection often occurring in immunocompromised patients1,2). Despite the recognition of several species of the genus, the non-C. neoformans including C. albidus are generally regarded as non pathogenic saprophytes3). Infections due to other species of Cryptococcus are extremely rare and poorly substantiated.
There have only been 14 previously reported cases in which Cyptococcus albidus has been isolated as a pathogen, none of which occurred in a renal transplant recipient. To the best of our knowledge, this is the first reported case of disseminated cryptococcosis presenting cutaneous infection, septic shock, and acute respiratory distress syndrome (ARDS) caused by C.albidus in a renal transplant recipient. Our experience with this case is instructive since it was treated successfully with fluconazole alone despite the serious multiorgan involvement. We have reviewed and compared all known cases of infection with C. albidus.
A 23-year-old Asian man with chronic renal allograft dysfunction was admitted to our hospital in May 2001 with a 10-day history of fever, intermittent chills, dry cough and progressive dyspnea. He underwent renal transplantation in 1994 for end-stage renal disease of unknown cause diagnosed in 1993. He had been on a medication of cyclosporine 200 mg/day and prednisolne 5ŌĆō20 mg/day. His temperature was 40┬░C, blood pressure 120/70 mmHg supine, pulse rate 120/min and respiratory rate 20/min. Respiratory examination revealed mildly decreased breathing sounds on the right lower chest. We also noted several erythematous tender macules measuring 0.5 cm in diameter on both shins. He had no neurological signs of meningitis. Chest radiograph obtained on admission demonstrated increased opacity on the right lower lobe. Arterial blood gas analysis on room air revealed a pH of 7.38, PaCO2 22.1 mmHg, PaO2 49.4 mmHg, and SaO2 85.3%. A complete blood count yielded a leukocyte count of 5,600/mm (91.6% neutrophils), hemoglobin 5.6 g/dL, and platelet count 267,000/mm3. The renal allograft function was in acute exacerbation on chronic dysfunction; BUN 113 mg/dL, Cr 8.5 mg/dL, Na 125 mmol/L, K 6.4 mmol/L, protein 5.2 g/dL, albumin 2.9 g/dL, glucose 102 mg/dL. The patient had been treated with empirical antibiotics for the presumed diagnosis of severe community-acquired pneumonia. On the next day, his respiratory status deteriorated dramatically with a rapid development of hypotension. A repeat chest radiograph showed nearly complete opacification of both lung fields (Figure 1). The patient was intubated and placed on mechanical ventilation. Dobutamine and dopamine were administered. Urgent anti-CMV IgM, CMV PCR and anti-HIV were negative. Anti-mycoplasma antibody titer was <1:20. Acid-fast bacillus smear and methenamine silver stain of sputum yielded negative results. The initial three sputum and blood cultures were sterile. On the eighth hospital day, tender macules on both shins coalesced to form erythematous patches. Skin biopsy showed granulomatous inflammation in the dermis and numerous yeast organisms with clear thick capsules (Figure 2). The cerebrospinal fluid (CSF) exam was clear with normal glucose and slightly increased protein (95 mg/dL). The cryptococcal antigen titer was elevated in CSF at >1:256 and at >1:516 in serum. However, the microscopic examination of CSF preparations with India ink was negative for encapsulated yeasts and CSF cultures were negative. The culture of skin biopsy isolated a yeast organism, which was identified as Cryptococcus albidus by characteristic morphology, fermentation, and carbon assimilation tests using the API 20c AUX system (bioM├®rieu, Marcy-lŌĆÖEtoile, France). The patient was treated intravenously with fluconazole immediately after the skin biopsy. After 10 days of fluconazole therapy, his chest radiograph and CT scan showed marked clearing with only one cavitary nodular lesion on the left upper lobe (Figure 3). Percutaneous needle aspiration was performed for the left upper pulmonary nodule. Cytologic examination of the aspirates also revealed a typical morphology of the numerous cryptococci (Figure 4). The patient was discharged on an oral regimen of fluconazole (200 mg/day). Fluconazole maintenance therapy was continued for 12 months on a long-term basis for prevention of cryptococcosis. At the time of the most recent follow-up, July 2002, his chest radiograph was stable and we detected no evidence of recurrent cryptococcal infection.
Cryptococcosis has been the most common cause of life-threatening opportunistic fungal infection among immunocompromised patients, and its incidence ranges between 8% and 10% in HIV-infected patients and between 1% and 5% in organ transplant recipients2).
Cryptococus albidus is traditionally considered a non-pathogenic yeast. There have been only 14 previously reported cases of systemic disease in which this opportunistic organism was isolated and implicated as a pathogen: 6 cases of meningitis, 5 cases of fungaemia, 1 case of peumonitis, 1 case of pleural infection, 1 case of direct skin infection (Table 1). However, C.albidus has never before been reported as a disseminated form of cryptococcocal infection nor as a pathogen in a renal transplant recipient. Septic shock is a very uncommon complication of this infection, with two cases having been reported previously, only in disseminated cryptococcosis caused by Cryptococcus neoformans1). Disseminated cryptococcosis has a mortality rate higher than 80% when associated with respiratory failure. Recent reports have highlighted diagnostic delay as a major factor contributing to its high associated mortality4).
In this case, cryptococcal antigenic titer was considerably high in serum and CSF despite the isolation of C.albidus. Although C. albidus shares many biochemical characteristics and so\me capsular antigens with C.neoformans, the cryptococcal antigen latex agglutination testing has been reported to yield negative results in many cases of C. albidus infection5). The definitive diagnosis thus rests on India ink staining, fungal culture and identification by routine biochemical test to confirm the presence or absence of this organism6,7). However, Miyagawa et al. have previously observed that most of the anti-C.neoformans IFA antibody in serum was absorbed with C. albidus, and vice versa8). C.albidus strains have their cell surface antigenic factors are identical to those of C.neoformans serotype A, with no more specific antigens in addition to the antigens shared with the latter8).
We performed percutaneous needle aspiration of the pulmonary nodule to prove C.albidus to be the pathogen causing both the lung lesion as well as skin infection. However, isolation of C.albidus with specimen culture was not able to be performed due to a laboratory fault. We could only identify numerous cryptococcus on slides with pulmonary aspirate. Thus, there is both possibility of the coinfection of C.neoformans with C.albidus and isolated C.albidus infection, which cause cryptococcal antigen titers to increase. ARDS developed in this patient might be secondary to sepsis but fungal invasion into the lung tissue was identified by percutaneous needle aspiration cytology.
To date, the mainstay of treatment for systemic cryptococcosis is known to be a combination therapy of intravenous amphotericin B with or without flucytosine followed by fluconazole9). Immunocompromised patients, such as solid organ transplantation recipients, require more prolonged therapy. Moreover, recent evidences support the necessity for life-long maintenance therapy of fluconazole given indefinitely for secondary prophylaxis since there is more than a 50% relapse rate after an apparently successful treatment of both meningeal and extrameningeal diseases10). However, the administration of amphotericin B frequently results in the rapid onset of renal dysfunction, necessitating dosage reduction or discontinuation of therapy. Renal transplant recipients are particularly susceptible to the nephrotoxicity of amphotericin B since its concomitant use with cyclosprin or taclolimus can precipitate renal failure2). Most of the previous 14 patients with C.albidus infection, including 5 of the 9 survivors, were treated with amphotericin B. The patients treated with other regimens also survived, including 1 patient with oral ketoconazole, 1 patient with oral azithromycin and paromycin and 2 patients with fluconazole. There has been a report on the successful therapy of C.albidus septicaemia in a HIV patient with oral fluconazole monotherapy11). Fluconazole seems to be substitutable for amphotericin B as an initial antifungal agent for cryptococcosis. Given our success in this anecdotal case, fluconazole monotherapy warrants study in future controlled trials for the treatment of disseminated cryptococcosis by non-neoformans species in transplant recipients.
This case emphasizes the importance of considering unusual emerging cryptococcal as well as other fungal infections and suggests that C.albidus must be added to the increasing number of recently described causative agents of fungal infections in critically ill immunocompromised patients such as transplant recipients.
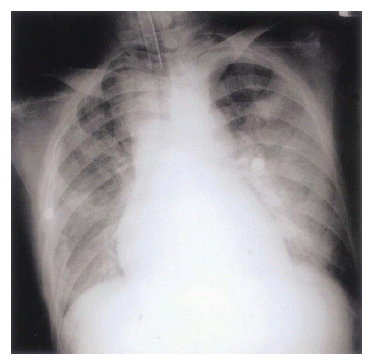
Figure┬Ā1.
Chest X-ray on second hospital day, shows rapidly aggravated bilateral airspace consolidation.
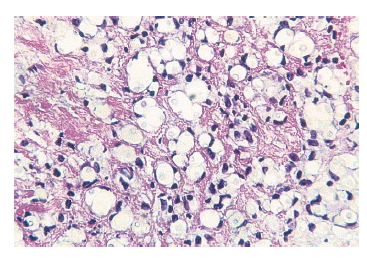
Figure┬Ā2.
Skin biopsy showing numerous encapsulated yeast-like organisms surrounded by large clear spaces and inflammatory cells consisting of lymphoid cells in subcutis (H&E stain, ├Ś400).
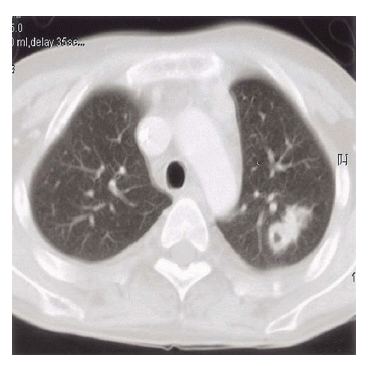
Figure┬Ā3.
Chest CT showed a irregular, spiculated nodule with cavity at the apicoposterior segment of the left upper lobe.
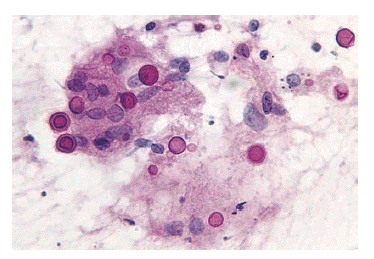
Figure┬Ā4.
High power microscopic finding of fine needle aspiration specimen of the pulmonary nodule shows scattered round to ovoid cryptococcus with polysaccharide capsules and occasional budding with inflammatory cells consisting of histiocytes and a few giant cells (PAS stain, ├Ś400).
Table┬Ā1.
Feature of the 14 Known Cases of Cryptococcus albidus infection
REFERENCES
1. Loranzo F, Gomez-Mateos J, Irles JA, Mrendez B, Martin E. Fulminant septic shock in AIDS patients caused by disseminated cryptococcosis. Eur J Clin Microbiol Infect Dis 18:151ŌĆō1521999.


2. Singh N, Gayowski T, Marino IR. Successful treatment of disseminated cryptococcosis in a liver transplant recipient with fluconazole and flucytosine, an all oral regimen. Transpl Int 11:63ŌĆō651998.


3. Kordossis T, Avlami A, Velegraki A, Stefanou I, Georgapoulos G, Papalambrou C, Legakis NJ. First report of cryptococcus laurentii meningitis and a fatal case of cyptococcus albidus cryptococcaemia in AIDS patients. Med Mycol 36:335ŌĆō3391998.


4. Visnegarwala F, Graviss EA, Lacke CE, Dural AT, Johnson PC, Atmar RL, Hamill RJ. Acute respiratory failure associated with cryptococcosis in patients with AIDS. Clin Infect Dis 27:1231ŌĆō12371998.


5. Horowitz ID, Blumberg EA, Krevolin L. Cyptococcus albidus and mucormycosis empyema in a patient receiving hemodialysis. South Med J 86:1070ŌĆō10721993.


6. Narayan S, Batta K, Colloby P, Tan CY. Cutaneous cryptococcus infection due to C.albidus associated with Sezary syndrome. Br J Dermatol 143:632ŌĆō6342000.


7. Wells GM, Gajjar A, Pearson TA, Hale KL, Shenep JL. Pulmonary cryptosporidiosis and cryptococcus albidus fungemia in a child with acute lymphocytic leukemia. Med Pediatr Oncol 31:544ŌĆō5461998.


8. Miyagawa T, Hamagami S, Tanigawa N. Cyptococcus albidus-induced summer-type hypersensitivity pneumonitis. Am J Respir Crit Care Med 161:961ŌĆō9662000.


9. Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, Sobel JD, Dismukes WE. Practice guidelines for the management of cryptococcal disease. Clin Infect Dis 30:710ŌĆō7182000.


-
METRICS

- Related articles
-
Recurrent neutropenia induced by rifabutin in a renal transplant recipient2014 July;29(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


