 |
 |
| Korean J Intern Med > Volume 19(1); 2004 > Article |
|
Abstract
Background :
Pulmonary aspergilloma usually results from the ingrowth of colonized Aspergillus from a damaged bronchial tree, a pulmonary cyst, or from the cavities of patients with underlying lung diseases. In the present study, we analyzed the clinical features, diagnostic methods, and managements of 36 patients with pulmonary aspergilloma.
Methods :
Thirty-six patients were diagnosed as having pulmonary aspergilloma at Chung-Ang University Hospital between February 1988 and February 2000. Their medical records were reviewed retrospectively.
Results :
The age of patients (median±SD) was 53.3±11.8 years, the male to female ratio was 2.36:1, and the most frequent symptom was hemoptysis, which occurred in 24 patients (65%). The most common underlying disease was pulmonary tuberculosis (81%), and the upper lobes of both lungs were the most frequently involved sites. Nine patients received a chest CT in the prone position and seven of these showed a movable fungus ball. Eleven patients were positive for the precipitin antibody to A. fumigatus. Twenty patients underwent surgical resection, and post-operative complications were reported in seven cases. The post-operative mortality was 5.6% (2/36).
Conclusion :
Pulmonary aspergilloma usually develops in the patients with underlying lung diseases. Resectional lung surgery is considered the mainstay of therapy for pulmonary aspergilloma. However, this operation is associated with significant complications and death in some cases. Therefore, it is necessary to develop reasonable criteria for selection of candidates for such surgery.
Pulmonary aspergillosis, which is caused by Aspergillus fumigatus, can be classified into 5 categories according to its pathophysiology, clinical manifestation, and treatment modality. Allergic bronchopulmonary aspergillosis (ABPA) is characterized by peripheral blood eosinophilia in patients with bronchial asthma. Aspergilloma develops when aspergillus colonizes and grows inside lung cavities and forms a ball-like structure. Chronic necrotizing aspergillosis is a locally invasive form of aspergillosis, which involves surrounding lung parenchyme and pleura. When the systemic inflammatory form of aspergillosis develops in immunocompromised hosts, it is called invasive aspergillosis. Finally, hypersensitiveity pneumonitis may develop after the inhalation of organic particles contaminated by aspergillus1).
Aspergilloma is composed of hyphae of Aspergillus, fibrin, mucus, inflammatory cells, blood, and epithelial cell components2, 3). Many cavitary lung diseases -tuberculosis, sarcoidosis, cavitary tumor, pulmonary fibrosis, bronchiectasis, and histoplasmosis - are complicated by aspergilloma. Among these, pulmonary tuberculosis is the most common cause of cavities facilitating the development of aspergilloma2). Around 11–17% of the cavitary forms of pulmonary tuberculosis have been reported to be complicated by aspergilloma5). It has also been reported that aspergilloma can develop in otherwise healthy lungs. For example, Aspergillus species colonizing the respiratory tract can secrete digestive enzymes into the surrounding lung parenchyme and create space for the growth of the fungus ball6).
The clinical manifestations of pulmonary aspergilloma are diverse, ranging from asymptomatic cases to massive and sometimes fatal hemoptysis9). Therefore, optimal therapeutic modalities for aspergilloma are dependent upon clinical presentations. In some cases of aspergilloma, natural dissolution could occur with the disappearance of clinical symptoms4). However, in another disease spectrum, emergent thoracotomy is required because of massive hemoptysis. In the present study, we performed a retrospective analysis upon 36 patients with pulmonary aspergilloma, with a focus on the treatment modalities used and their outcomes.
This study was based on the medical records of 36 pulmonary aspergilloma patients, from Chung-Ang University Hospital between February 1988 and February 2000. A diagnosis of pulmonary aspergilloma was made on the basis of pathologic examination of biopsied or resected lung specimens in 26 cases. In 10 cases, the diagnosis was made on the basis of characteristic radiological manifestations, such as the air-meniscus-sign or the movability of the fungus ball in the prone position on Chest CT10, 11). All patients with a radiology-based diagnosis showed a positive result for precipitating antibody against aspergillus or a positive sputum culture for aspergillus on at least two occasions. We evaluated demographic data, clinical manifestations, treatment modalities, and the outcomes for selected patients using the above criteria.
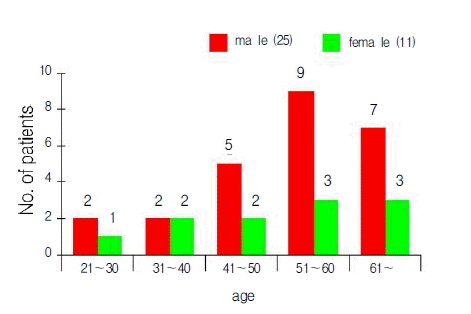
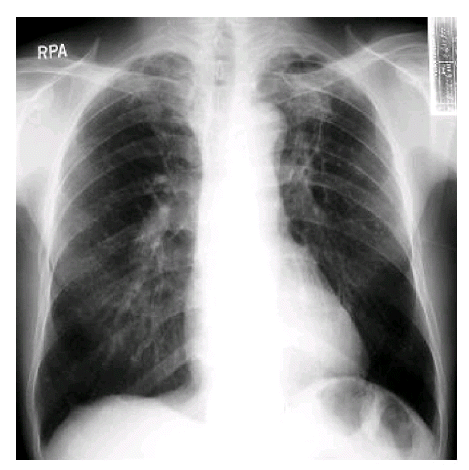
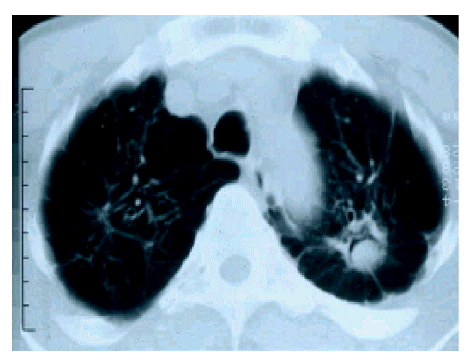
The ages of patients ranged from 21 to 65 years (median age±SD; 53.3±11.8), and there were 25 males and 11 females (M:F=2.36:1) (Figure 1). The chief complaints at the time of visit were hemoptysis (26 cases, 72%), cough, and dyspnea. Pulmonary tuberculosis was the most common underlying disease (24 cases, 66%), followed by bronchiectiasis (3 cases). There were four cases of aspergilloma unaccompanied by underlying lung diseases. Patients with pulmonary aspergilloma also suffered from other chronic non-pulmonary diseases, such as type 2 diabetes mellitus (2 cases) and chronic hepatitis C (1 case). The time interval from the diagnosis of tuberculosis to the manifestation of aspergilloma varied from less than 1 year to over 10 years. The right upper lobe was the most frequent site of involvement for pulmonary aspergilloma (15 cases), followed by the left upper lobe (12 cases). This site preference of aspergilloma could be explained by the fact that both upper lobes are also the most common sites of involvement for cavitary pulmonary tuberculosis (Figure 2). The air-meniscus sign by radiography is one of the characteristic findings of pulmonary aspergilloma, and was found in 16 of the 36 cases enrolled in the present study (44%). In addition, prone position chest CT was performed in 9 cases and 7 (78%) showed a fungus ball that moved on changing position (Figure 3).The size of the cavities varied from 1 cm to 5 cm in diameter. However, no significant correlation was found between the size of the cavity and the amount or frequency of hemoptysis. A sputum examination was performed in 31 cases, 6 of these (19%) showed repeated (atleast two) positive smear tests for fungal hyphae and there were 11 cases (36%) of documented A. fumigatus growth. Eleven of 17 patients (64%) had a positive serologic test against anti-A. fumigatus precipitating antibody.
As for treatment, surgery was performed in 20 cases (55%), bronchial artery embolization without surgical treatment was done in 4 cases (12%), and 12 cases (33%) received conservative medical treatment only. The pulmonary functions of patients that received surgery were better than those who did not. The forced expiratory volume in one second (FEV1) of patients with surgery was 2,473±977 mL (76% of the predicted value), while those that received non-surgical treatment had a mean FEV1 of 1,633±420 mL (67.6% of the predicted value) (p<0.05). Fifteen of the surgical cases (75%) showed and FEV1 of more than both of 70% of the predicted value and 1,000 mL in absolute amount. Eight non-surgical patients (50%) showed FEV1s 60% below the predicted value. This means that patients with a high reserve of pulmonary function were selected for surgical treatment. In terms of surgical methods, lobectomy was performed in 11 cases (55%), segmentectomy was done in three cases (15%), and combined lobectomy and segmentectomy was performed in four (20%). Combined lobectomy and wedge resection was the treatment of choice in one case, and wedge resection was used only in one case. The reported post-operative complications were bronchopulmonary fistula (2 cases), empyema (2 cases), pneumothorax (2 cases), and atelectasis (1 case). Two patients died of sepsis after surgery, so the post-operative mortality was 10%. There was no reported case of aspergilloma recurrence after surgery. In contrast, four of seven patients who received bronchial artery embolization as a first therapy redeveloped hemoptysis. One patient received three repeated bronchial artery embolization and the other three patients received one repeated embolization.
Ninety five percent of human infections by aspergillus are caused by Aspergillus fumigatus, making this the most invasive kind of Aspergillus species. In line with this, only one case of aspergilloma other than by A. fumigatus was found in the present study (which was by A. niger). Most patients with aspergilloma are known to be asymptomatic. Actually many cases are diagnosed incidentally during medical check-up. Even if there are symptoms, they usually are nonspecific, i.e., cough, dyspnea, and general weakness, and could be explained by the underlying lung diseases rather than the aspergilloma. However, because aspergilloma could manifest itself as massive, fatal hemoptysis, the attending physicians should be vigilant. In the present study, there were 26 cases of hemoptysis, which seems a higher prevalence than previously reported17). This high frequency of hemoptysis could be due to the fact that patients were registered at a university-affiliated referral hospital. Hemoptysis in aspergilloma seems to be caused by the mechanical irritation of the exposed vasculature inside the cavity by the rolling fungus ball. In addition, chemical agents such as, hemolytic endotoxin and trypsin-like proteolytic enzymes may also play some role in the development of hemoptysis12,13).
The air-meniscus sign is a well known characteristic radiologic finding in aspergilloma12. In addition, when the fungus ball shows mobility upon prone position chest CT, the probability of aspergilloma is higher11). In line with this, we confirmed the air-meniscus sign in 44% of patients and mobility of fungus ball on prone position chest CT in seven of nine cases (78%). However, it must be noted that absence of characteristic signs does not rule out the possibility of aspergilloma, and therefore, other diagnostic modalities (e.g., sputum fungus staining/culture, or serologic testing) should also be used to ensure the diagnosis of aspergilloma. Furthermore, air-meniscus sign can be observed in other conditions such as candidiasis, coccidioidosis, necrotizing lung cancer, lung abscess, and norcardiasis. Therefore, the differential diagnosis of pulmonary diseases with an air-meniscus sign is not always easy, and requires both experience and a broad knowledge of pulmonary diseases. One more notable radiologic feature was that both upper lobes were the most frequent sites of involvement by aspergilloma. This may be because cavitary pulmonary tuberculosis, which is the most common underlying disease for aspergilloma, also usually develops in both upper lobes.
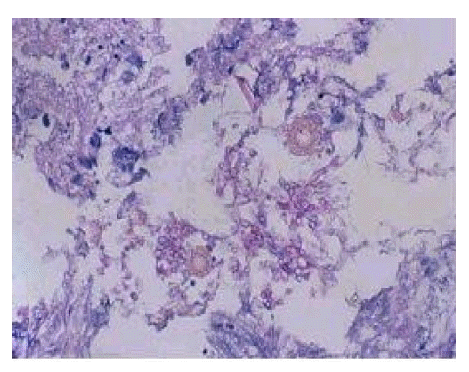
Sputum is easily contaminated by saprophytic fungi, therefore, only repeated bouts of positive results for fungal hyphae should be accepted as a meaningful sign for the diagnosis of aspergilloma2). In the present study, we confirmed repeated positive results of 58% for sputum smear testing of fungal hyphae, which means that sputum study is a valuable supportive technique for the diagnosis of aspergilloma. Another valuable marker for the diagnosis of aspergilloma is the detection of precipitating antibody to A. fumigatus by serologic testing. In our evaluation, the observed positive rate of 64% was lower than the previously reported rate of 90%. Although it was not easy to determine the exact reasons for the low positive rate observed in our patients, generally false negative results were associated with decreased immunity of patients and/or a reduced A. fumigatus antigenicity due to the extinction of viable organisms. The final confirmation of aspergilloma can be made by pathologic examination of resected lung specimens. On gross examination, aspergilloma looks like a brownish clay ball (Figure 4) that can be microscopically confirmed as aspergilloma by observing hyphae branching in acute angles (Figure 5).
In patients with aspergilloma and hemoptysis, surgical resection of the involved lung segment is the treatment of choice14–17). However, decision making of surgical resection is often hampered by poor reserve pulmonary function in patients with aspergilloma. In our study, patients who received surgical treatment had more favorable preoperative pulmonary function than those who did not (FEV1 2,473 vs. 1,353 mL). Moreover, no patient who received surgical resection suffered aspergilloma recurrence. Therefore, patients with aspergilloma suffering from repeated bouts of hemoptysis should be considered as candidates for surgical treatment, provided their pulmonary functions lie within the acceptable range. Repeated bronchial artery embolizations represent the best therapeutic modality for patients with low pulmonary function reserves. However, bronchial artery embolization is often followed by collateral vessel formation, which could provide a nidus for the recurrence of hemoptysis18–20). Modified methods for treating repeated hemoptysis by aspergilloma have also been reported. Ramirez et al. achieved good outcomes using transbronchial catheter instillation of amphotericin-B and potassium iodide into aspergilloma laden cavities21). Hargis et al. argued that the direct instillation of amphotericin-B into affected cavities should be considered in patients with poor lung function22). Although their reports have been followed by several similar studies in Korea, the long-term effects of these treatments need to be examined before they can be approved23, 24).
REFERENCES
1. Murray JF, Nadel JA, Mason RJ, Boushey HA. Textbook of respiratory medicine. 3th ed. 1131. Philadelphia: WB Saunders, 1999.
2. Glimp RA, Bayer AS. Pulmonary aspergilloma: diagnostic and therapeutic considerations. Arch Intern Med 143:303–3081983.


3. Daly RC, Pairolero PC, Piehler JM, Trastek VF, Payne WS, Bernatz PE. Pulmonary aspergilloma: results of surgical treatment. J Thorac Cardiovasc Surg 92:981–9881986.


4. Hammerman KJ, Christianson CS, Huntington I, Hurst GA, Zelman M, Tosh FE. Spontaneous lysis of aspergillomata. Chest 64:679–6791973.


5. Research Committee of the British Thoracic and Tuberculosis Association. Aspergilloma and residual tuberculosis cavities: the result of a resurvey. Tubercle 51:227–2451970.


6. Kibbler CC, Milkins SR, Bhamra A, Spiteri MA, Noone P, Prentice HG. Apparent pulmonary mycetoma following invasive aspergillosis in neutropenic patients. Thorax 43:108–1121988.



7. Yu HS, Kim BY, Suh CH, Nam CH, Yoo BH, Lee JH. Surgical treatment of pulmonary aspergillosis. Korean J Thorac Cardiovasc Surg 17:269–2741984.
8. Lee CK, Park SI, Suh JJ, Won JH. Surgical analysis of pulmonary aspergilloma. Korean J Thorac Cardiovasc Surg 33:245–2512000.
9. Kang TK, Kim CH, Park JY, Jung TH, Sohn JH, Lee JH, Han SB, Jeon YJ, Kim KB, Chung JH, Lee KH, Lee HW, Shin HS, Lee SC, Kweon S. Clinical chracteristics of pulmonary aspergilloma. Tuberc Respir Dis 44:1308–13171997.

12. Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of prognosis in relation to hemoptysis and survey of treatment. Thorax 38:572–5781983.



13. Pimentel JC. Pulmonary calcification in the tumor-like form of pulmonary aspergillosis: pulmonary aspergilloma. Am Rev Respir Dis 94:208–2161966.

14. Chen JC, Chang YL, Luh SP, Lee JM, Lee YC. Surgical treatment for pulmonary aspergilloma: a 28 year experience. Thorax 52:810–8131997.



15. Research Committee of the British Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. Tubercle 49:1–111968.


16. Solit RW, Mckeown JJ Jr, Smullens S, Fraimo W. The surgical implications of intracavitary mycetomas (fungus balls). J Thorac Cardiovasc Surg 62:411–4221971.


17. Rafferty P, Biggs BA, Crompton GK, Grant IW. What happens to patients with pulmonary aspergilloma?: analysis of 23 cases. Thorax 38:579–5831983.



18. Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest 97:541–5461990.


19. Kim BC, Kim JM, Kim YS, Kim SM, Choi WY, Lee KS, Yang SC, Yoon HJ, Shin DH, Park SS, Lee JH, Kim CS, Seo HS. Effect of bronchial artery embolization in the management of massive hemiptysis: factors influencing rebleeding. Tuberc Respir Dis 43:590–5991996.

20. Yoo BS, Rhyu JS, Lee WY, Song KS, Ahn KH, Yong SJ, Shin KC, Kim YJ. Transcatheter arterial embolization for hemoptysis. Tuberc Respir Dis 42:50–571995.

22. Hargis JL, Bone RC, Stewart J, Rector N, Hiller FC. Intracavitary amphotericin-B in the treatment of symptomatic pulmonary aspergillomas. Am J Med 68:389–3941980.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



