 |
 |
| Korean J Intern Med > Volume 19(1); 2004 > Article |
|
Abstract
Background :
Since pancreatic cancer metastasizes early regardless of the size of the primary tumor, it is suggested that angiogenic factor is upregulated in this disease. Among the angiogenic factors, vascular endothelial growth factor (VEGF) is the most potent and specific growth factor. The aim of this study is to elucidate the prognostic value of VEGF expression in pancreatic cancers.
Methods :
We analyzed the VEGF expression using immunohistochemistry in 72 resected pancreatic ductal adenocarcinomas. We examined the prognostic value of the VEGF expression along with its relationship with the clinicopathological features.
Results :
VEGF expression and mutant p53 expression were not associated with microvessel density. VEGF expression was positively associated with mutant p53 expression. There were no statistically significant relationships between the VEGF expression and other clinicopathological features, such as age, sex, CA19-9, tumor size, location, tumor differentiation, and stage. VEGF expression was not associated with patient survival.
Pancreatic cancer is one of the most common and lethal cancers in the world with a median survival time of approximately 3 months1). Although combined chemoradiation results in improvement of local control, it has only a modest impact on survival due to the development of distal metastases1). Pancreatic cancer can entail the substantial development of new blood vessels within the tumor tissue, and it is known that the growth and progression of solid tumors depend on such angiogenesis2). Among the proangiogenic factors, the vascular endothelial growth factor (VEGF) is the most potent and specific growth factor3, 4). There are discrepancies about the prognostic value of the VEGF in pancreatic cancer. A previous report demonstrated that VEGF expression was an independent prognostic factor in pancreatic cancer5). There were somewhat contradictory results that the VEGF expression did not correlate with various clinicopathological parameters such as the vessel count, metastasis, recurrence and survival6). To estimate the usefulness of the prognostic factor, many molecular and biological markers were investigated. This study was conducted to elucidate the prognostic value of VEGF expression as well as evaluate its relationship with clinicopathological variables in pancreatic ductal adenocarcinoma.
Samples of 72 pancreatic ductal adenocarcinomas were obtained at the time of surgical resection from Samsung Medical Center (Seoul, Korea) between January 1995 and September 1999. The age of patients ranged from 16 to 79 years with a median age of 59.8 years. There were 43 male and 29 female patients. The median period of follow-up was 12.6 months (range, 1ŌĆō66.2 months). The median survival time was 353 days. Follow-up data were gathered by reviewing of the patientŌĆÖs charts. The patientsŌĆÖ outcomes were verified and updated through the medical record departments of Samsung Medical Center and the telephone. Fifty-six (77.8%) patients died, and 16 patients were still alive during the follow-up periods. Twenty-six patients received adjuvant chemotherapy. Tissues were fixed in 10% formalin for 12 hours and embedded in paraffin. Tissues were stained with hematoxylin and eosin. The histopathologic features of the pancreatic ductal adenocarcinoma were examined for the following: tumor size, location, tumor differentiation, and stage. The tumor stage was determined according to the staging manual of the American Joint Committee on Cancer7).
The representative tissue sections including both an adequate amount of tumor tissue and the adjacent non-tumor epithelial cells were selected and sectioned in 4-╬╝m-thickness. Immunohistochemical study was performed using the streptavin-biotin complex method. The primary antibodies used and working dilutions employed were as follows: VEGF (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA, 1:2000), CD 34 (Immunotech, Marselle Cedex, France,1:50),and p53 (Zymed, San Francisco, CA, USA, 1:80 dilution).For antigen retrieval, the sections were pretreated in an 800-W microwave oven in a 10 mM citrate buffer of pH 6.0 for 10 minutes. The sections were incubated with the primary antibody for 10 minutes, and then, the secondary antibody and the streptavin-peroxidase complex (LSAB kit, Dako, CA, USA) were applied, sequentially. DAB (3,3ŌĆ▓-diaminobenzidine tetrahydrochloride) was used as a chromogen, and MayerŌĆÖs hematoxylin counterstain was applied. Negative controls were run simultaneously with an omission of the primary antibody. The proportion of VEGF positive tumor cells was quantified and was assigned to one of two categories; 0, 10% : 1, >10%3). Microvessel density was recorded by counting the CD 34 positive vessels in the highest vascularized area in X200 fields (a X10 ocular and a X20 objective lens)8). Vessels of a caliber larger than approximately eight RBCs, vessels with thick muscular walls, and vessels in sclerotic areas were excluded from the count. The counts were expressed as the total number of microvessels per mm2 in X200 fields using the image analysis system (CIRES program, IBAS model, Zeiss, Germany). Mutant p53 immunostaining was scored as follows. The proportion of positive tumor cells was quantified and was assigned to one of two categories; 0, 10% : 1, >10%9). The stained slides were independently evaluated by two authors (W.O.J. and S.Y.S), and the difference in interpretation was resolved by a consensus.
The correlation between VEGF expression and clinicopathological features (age, sex, CA19-9, tumor size, location, tumor differentiation, stage, CD 34 and mutant p53) was determined by the SpearmanŌĆÖs rank correlation. A Cox proportional hazards model for the risk ratio was used to assess the simultaneous contribution of the following baseline covariates: age, sex, CA19-9, tumor size, location, tumor differentiation, stage, CD 34, mutant p53, and VEGF. Survival curves were constructed using the method of Kaplan-Meier. A probability of p<0.05 was considered statistically significant.
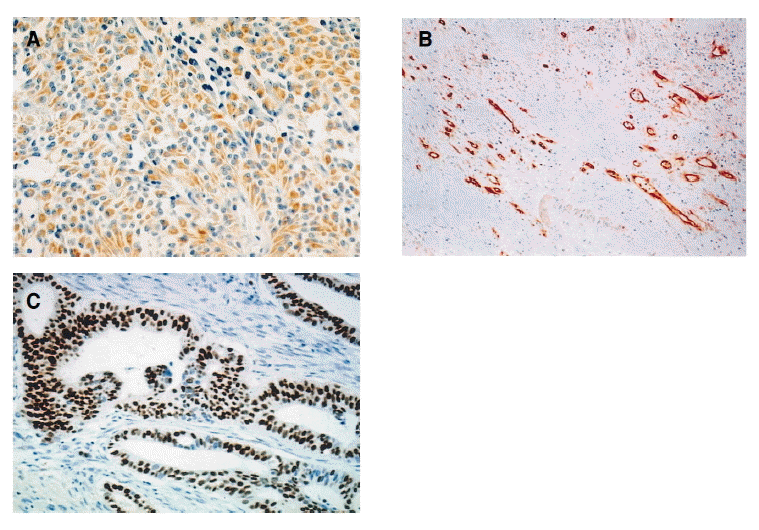
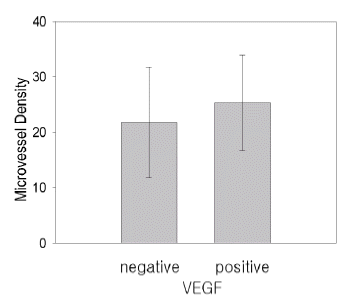
VEGF immunoreactivity was present in 23/72 tumors (32%) (Figure 1A), and The mutant p53 expression was present in 44/72 tumors (61%) (Figure 1C). The VEGF expression was not associated with microvessel density (p=0.059) (Figure 1B, 2), and microvessel density was not associated with the mutant p53 expression (p=0.09). VEGF expression had a positive correlation with the p53 mutation (p=0.049). When the relationship between the VEGF expression and other clinicopathological parameters were evaluated, the statistical analysis revealed no significant correlation with other clinicopathological parameters (Table 1).
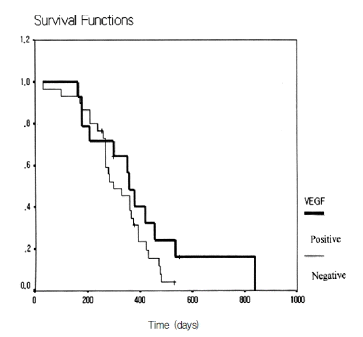
Multivariate analysis was performed according to the Cox proportional hazard model in order to evaluate the prognostic value of the VEGF expression, microvessel density, and mutant p53 expression. VEGF expression (p=0.239), microvessel density (p=0.398), and mutant p53 expression (p=0.772) did not predict unfavorable prognosis independently of other clinicopathological features (Table 2). The survival curves for the patients as a function of the VEGF expression are shown in Figure 3. It was illustrated using the Kaplan-Meier survival curve p=0.448) that VEGF expression was not associated with patient survival.
A reason for the poor prognosis in pancreatic cancer is the development of early metastasis regardless of the primary tumor growth1). Angiogenesis is an integral part of the cascade of biologic events involved in tumor metastasis10). If angiogenesis is essential for tumor growth and metastasis, there may be differences in the quantitative assessment of vascular proliferation between tumors with different prognostic features10, 11) Increased angiogenesis in pancreatic cancer is related to cancer aggressiveness2, 5). To date, many angiogenic factors for pancreatic cancers have been reported, such as the transforming growth factors (TGF)-╬▒, TGF-╬▓, aFGF (acidic fibroblast growth factor), bFGF (basic fibroblast growth factor), angiogenin, VEGF, and PD-ECGF (platelet-derived endothelial cell growth factor)5, 12, 13). Among the angiogenic factors, VEGF is the most potent and the most specific growth factor13). Since pancreatic cancer metastasizes early regardless of the size of the primary tumor, it was expected that VEGF was upregulated in this disease3). But, there are many controversies about the prognostic value of VEGF in pancreatic cancer. The previous report showed that strong VEGF immunoreactivity was present in the cancer ceu in 64% of the pancreatic cancer tissues. and the presence of VEGF was associated with an increased blood vessel number, large tumor size, and enhanced local spread but was not associated with a reduction in patient survival time3). In our study, the VEGF expression was neither associated with an increased blood vessel number nor patient survival. Dilution, incubation time, and method of interpretation were somewhat different in the previous reports3ŌĆō5, 13). Most of all, the analysis of the large sample size by immunohistochemitry combined with other objective experimental methods, such as Northern blot and real time PCR, was needed to elucidate the exact prognostic value of the VEGF in pancreatic ductal adenocarcinoma.
p53 mutations might be acquired in the later stages associated with metastatic progression and higher proliferation activity14, 15). It was suggested that the mutant p53 gene was a potent stimulant of VEGF and was related to tumor angiogenesis14). Mutant p53 expression may be a beneficial prognostic factor in pancreatic cancers9). The previous report showed that mutations in the p53 gene occurred in approximately 60% of the tumors and appeared to be an independent prognostic factor for patient survival1, 9). The present study demonstrated that mutations in the p53 gene occurred in 61% of the tumors and was not a beneficial prognostic factor for patients with pancreatic cancer. The positive VEGF expression was associated with a poor prognostic factor such as mutant p53.
The only limitation of our study are the immunohistochemistric results. To know the exact prognostic value of VEGF, analysis of large sample size by immunohistochemistry, as well as the quantitative assay, such as the Northern blot, needs to be elucidated. The present study demonstrated that VEGF expression was neither associated with microvessel density nor patient survival in pancreatic ductal adenocarcinoma. This study suggests that it is difficult for the VEGF expression to be used as a prognostic marker in clinical practice.
Figure┬Ā1.
Immunohistochemical analysis (A) VEGF immunostaining of pancreatic ductal adenocarcinoma. Cytoplasms of the tumor cells are stained positive (H&E, X200). (B) Immunostaining of CD 34 with high microvessel density (H&E, X40). (C) Mutant p53 immunostaining of pancreatic ductal adenocarcinoma. The nucleuses of the tumor cells are stained positive (H&E, X100).
Figure┬Ā2.
The correlation between VEGF expression and microvessel density. VEGF expression was not associated with microvessel density (p=0.059).
Figure┬Ā3.
The effect of VEGF immunostaining on cumulative survival rate after the surgical resection of pancreatic ductal adenocarcinomas. Positive VEGF expression did not predict short survival (p=0.448).
Table┬Ā1.
The relationship between VEGF immunostaining and other clinicopathological features of pancreatic ductal adenocarcinomas (SpearmanŌĆÖs rank correlation)
REFERENCES
1. van Riel JM, Giaccone G, Pinedo HM. Pancreaticobiliary cancer: the future aspects of medical oncology. Ann Oncol 10:296ŌĆō2991999.


2. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4ŌĆō61990.


3. Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 3:1309ŌĆō13161997.

4. Claffey KP, Robinson GS. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev 15:165ŌĆō1761996.


5. Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer 79:1553ŌĆō15631999.



6. Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 34:1439ŌĆō14471998.


7. Fleming D, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, OŌĆÖSullivan B, Sobin LH, Yabro JW. Amercian Joint Committee On Cancer, Cancer Staging Manual. 121ŌĆō126Philadelphia: Lippincott-Raven, 1997.
8. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med 324:1ŌĆō81991.

9. Barton CM, Staddon SL, Hughes CM, Hall PA, OŌĆÖSullivan C, Klppel G, Theis B, Russell RC, Neoptolemos J, Williamson RC. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer 64:1076ŌĆō10821991.



10. Ozer E, Ozkal S, Karademir S, Sagol O, Sokmen S, Coker A, Kpelioglu A, Astarciolu I. Angiogenesis and p53 and H-ras mutations in pancreatic ductal adenocarcinoma. Anal Quant Cytol Histol 21:473ŌĆō4761999.

11. Shimoyama S, Kaminishi M. Increased angiogenin expression in gastric cancer correlated with cancer progression. J Cancer Res Clin Oncol 126:468ŌĆō4742000.


12. Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, Kobrin MS, Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res 53:5289ŌĆō52961993.

13. Mineta H, Miura K, Ogino T, Takebayashi S, Misawa K, Ueda Y, Suzuki I, Dictor M, Borg A, Wennerberg J. Prognostic value of vascular endotheial growth factor (VEGF) in head and neck squamous cell carcinomas. Br J Cancer 83:775ŌĆō7812000.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



