 |
 |
| Korean J Intern Med > Volume 35(1); 2020 > Article |
|
Abstract
Background/Aims
For metastatic renal cell carcinoma (RCC), various prognostic scoring systems have been developed. However, owing to the low prevalence of nonclear cell RCC, the three most commonly used tools were mainly developed based on patients with clear cell histology. Accordingly, this study applied three prognostic models to Korean non-clear cell RCC patients treated with first-line temsirolimus.
Methods
This study analyzed data for 74 patients with non-clear cell RCC who were treated with temsirolimus as the first-line therapy at eight medical centers between 2011 and 2016. The receiver-operating characteristic (ROC) curves for the different prognostic models were analyzed.
Results
Twenty-seven (36.5%), 24 (32.4%), and 44 patients (59.5%) were assigned to the poor prognosis groups of the Memorial Sloan-Kettering Cancer Center (MSKCC), International Metastatic RCC Database Consortium (IMDC), and Advanced Renal Cell Carcinoma (ARCC) risk stratification models, respectively. All three prognostic models reliably discriminated the risk groups to predict progression-free survival and overall survival (p < 0.001). The area under the ROC curve (AUC) for progression and survival was highest for the ARCC model (0.777; 0.734), followed by the IMDC (0.756; 0.724) and the MSKCC (0.742; 0.712) models. Furthermore, the sensitivity and specificity for predicting progression were highest with the ARCC model (sensitivity 63.6%, specificity 85.7%), followed by the MSKCC (sensitivity 58.2%, specificity 86.5%) and the IMDC models (sensitivity 56.4%, specificity 85.7%).
Renal cell carcinoma (RCC) accounts for 2% to 3% of all malignant disease in adults, representing the seventh most common cancer in men and tenth most common cancer in women in the United States [1]. In Korea, RCC holds the tenth rank of incidence, regardless of age and sex, and the burden of RCC will continue to grow as Korea’s population ages [2]. The current World Health Organization classification system for RCC includes various tumor subtypes, where the major histologic variants are clear cell RCC (75% to 90% of tumors), papillary RCC (10% to 15%), and chromophobe RCC (4% to 5%) [3,4]. In the case of clear cell RCC, the von Hippel Lindau (VHL) gene is inactivated in 80% to 90% of patients [5]. Plus, since the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin pathways are important for this subtype, most previous randomized phase III clinical trials of targeted therapy have focused on patients with the clear cell histologic subtype [6-10]. Meanwhile, due to the relatively low prevalence of non-clear cell RCC, the optimal treatment and role of targeted agents for this patient group remain uncertain and under investigation. When Hudes et al. [10] compared the efficacy and safety of temsirolimus and interferon-α, they confirmed the benefit of temsirolimus for both poor-risk clear cell RCC and non-clear cell RCC. However, this is the only reported phase III trial that included patients with non-clear cell histology and these patients represented less than 20% of the total study group.
For metastatic RCC, various prognostic scoring systems have already been developed to define patient risk groups based on combining independent prognostic factors for survival outcomes [11]. The most widely used prognostic factor model is from the Memorial Sloan-Kettering Cancer Center (MSKCC) [12,13]. However, while the MSKCC model has been externally validated and is commonly used for clinical trial stratification, it is not as relevant for predicting survival in the era of immunotherapy [14]. Thus, in response to the need for new prognostic models in the era of VEGF-targeted therapies, Heng et al. [15,16] identified six prognostic factors: low hemoglobin level (< lower limit of normal), high level of corrected serum calcium (> 10 mg/dL), Karnofsky performance status (KPS, < 80%), a time from initial diagnosis to initiation of therapy of less than 1 year, high absolute neutrophil count (> upper limit of normal), and high platelet count (> upper limit of normal). This International Metastatic RCC Database Consortium (IMDC) model has already been proven useful as a prognostic tool in major clinical trials of novel targeted therapies [15,16]. Moreover, the IMDC model has even shown a better concordance index for patients with non-clear cell histologies [17,18]. Meanwhile, the Global Advanced Renal Cell Carcinoma (ARCC) pivotal study included six different variables: high serum lactate dehydrogenase (LDH) level (1.5 times the upper limit of normal), low hemoglobin level (< lower limit of normal), high level of corrected serum calcium (> 10 mg/dL), a time from initial diagnosis to initiation of therapy of less than 1 year, KPS (< 60%), and metastases in multiple organs [10].
Notwithstanding, the three most commonly used tools (MSKCC, IMDC, and ARCC models) were mainly developed based on patient cohorts with heterogeneous ethnic identity and clear cell histology treated with several targeted agents. Accordingly, this study compared the applicability of these three risk stratification models in the case of a homogenous Korean patient cohort treated with temsirolimus as the first-line therapy for non-clear cell RCC.
This study retrospectively reviewed the medical records of 74 patients treated with temsirolimus as the first-line therapy for non-clear cell RCC at eight medical centers between June 2011 and November 2016. The baseline demographic, clinicopathological, and laboratory data of the patients were collected retrospectively using uniform database templates to ensure consistent data collection. The collected data included the age, sex, performance status (PS), histologic subtype, serum LDH, corrected serum calcium, hemoglobin, white blood cell, platelets, initial date of diagnosis and treatment, status of nephrectomy, and metastasis. The status of organ metastasis and laboratory values were assessed at the time of diagnosis. Survival data were retrospectively collected from medical chart reviews. The tumor stage was determined according to the seventh edition of the Union for International Cancer Control/American Joint Committee on Cancer for RCC. The overall response rate (ORR) was defined as the proportion of patients with a best response of complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as the proportion of patients with a best response of CR, PR, or stable disease. Tumor response and progression were assessed using RECIST version 1.1 [19]. This study was approved by an Institutional Review Board of Kyungpook National University Chilgok Hospital (Daegu, Korea) (2017-12-017). Informed consent was waived by the board.
All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Descriptive statistics are reported as the proportion and median. Overall survival (OS) was defined as the time from the date of treatment to death from any cause, while progression-free survival (PFS) was defined as the time from the date of treatment to the date of any progression. The patient subgroups were compared in terms of PFS and OS using Kaplan-Meier curves, a logrank test, and multivariate survival analysis based on the Cox proportional hazard regression model. Hazard ratios and 95% confidence intervals (CIs) were estimated for each factor. All the tests were two-sided, and statistical significance was set at p < 0.05. A receiver-operating characteristic (ROC) curve analysis was conducted to evaluate the outcome predictive value of each risk model. All analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Table 1 shows the baseline characteristics of the 74 patients. The median age was 65 years (range, 36 to 85), and the number of male patients was 44 (55.9%). The non-clear cell RCC histologic subtypes were as follows: papillary (n = 28, 37.8%), unclassified (n = 19, 25.7%), chromophobe (n = 13, 17.6%), sarcomatoid (n = 3, 4.1%), microphthalmia transcription factor-family RCC translocation (n = 3, 4.1%), and collecting duct carcinoma of Bellini (n = 1, 1.4%). Thirty-four patients (45.9%) had a KPS under 80, and 29 (39.2%) had undergone prior nephrectomy. More than 70% (n = 55) of the patients had received treatment within 1 year of diagnosis, and 59 (79.7%) had multiple organ metastases. Seven patients (9.5%) showed neutrophilia, and 13 (17.6%) had thrombocytosis.
The ORR and DCR were 8.2% and 48.7%, respectively. CR was achieved in 1.3% (n = 1) of the patients, and 6.8% (n = 5) gained PR. At a median follow-up of 8 months (range, 4.7 to 11.3), 55 patients (74.3%) had experienced progression, and 39 (52.7%) had died. The 2-year OS and PFS rates were 7.5% and 29.2%, respectively (Fig. 1). In addition, Fig. 2 shows the survival rate according to the histologic subtype. The median OS was 34 months (95% CI, 16.2 to 41.2) for the chromophobe histologic subtype, 11 months (95% CI, 4.5 to 17.4) for the unclassified subtype, 8 months (95% CI, 5.6 to 10.3) for the papillary subtype, and 2 months (95% CI, 1.3 to 3.9) for the sarcomatoid subtype (p = 0.018). The median PFS for each histologic subtype were as follows: 13 months (95% CI, 3.6 to 23.6) for the chromophobe histologic subtype, 7 months (95% CI, 4.0 to 10.0) for the papillary subtype, 6.9 months (95% CI, 2.7 to 1.7) for the unclassified subtype, and 1 month (95% CI, 0 to 1.0) for the sarcomatoid subtype (p = 0.009).
Twenty-seven (36.5%), 24 (32.4%), and 44 patients (59.5%) were assigned to the poor prognosis groups of the MSKCC, IMDC, and ARCC risk stratification models, respectively (Table 2). The ARCC prognostic model only classified two patients (2.7%) in the low-risk group. The median number of cycles of temsirolimus for all patients was 22. The patients who were categorized into the poor prognosis groups underwent fewer cycles than those in the intermediate and favorable groups (9 cycles vs. 25 cycles vs. 70 cycles, respectively).
As shown in Figs. 3-5, the MSKCC, IMDC, and ARCC prognostic models exhibited a statistically significant link with PFS and OS (p < 0.001). In particular, the PFS rates for the ARCC risk groups were 29.5 (95% CI, 16.3 to 42.7), 10.2 (95% CI, 4.7 to 15.6), and 4.1 months (95% CI, 2.4 to 5.8) (p = 0.004). The ROC curves for the three prognostic models are shown in Supplementary Fig. 1. The area under the ROC curve (AUC) for progression and survival was highest for the ARCC model (0.777; 0.734), followed by the IMDC (0.756; 0.724) and MSKCC (0.742; 0.712) models (Table 3). Furthermore, the sensitivity and specificity for predicting progression were highest with the ARCC (sensitivity 63.6%, specificity 85.7%), followed by the MSKCC (sensitivity 58.2%, specificity 86.5%) and IMDC models (sensitivity 56.4%, specificity 85.7%) (Table 4).
Table 5 summarizes the results of the multivariate analysis of several prognostic factors in relation to OS and PFS. Three factors (KPS, anemia, and multiple organ metastases) were independent predictors of poor OS and PFS. Time from initial diagnosis to treatment < 1 year, neutrophilia, and thrombocytosis were significant prognostic factors for survival in univariate analysis, but not in multivariable analysis (Table 5).
This study comprehensively compared the prognostic utility of commonly used prognostic models in Korean non-clear cell RCC patients treated with temsirolimus as the first-line therapy. All the prognostic tools functioned well in terms of stratifying the non-clear cell RCC patients into risk groups with significantly different survival outcomes. In particular, the ARCC risk model showed relatively higher accuracy for predicting survival than the other two models. The non-clear cell RCC patients also showed inferior survival outcomes, consistent with previous studies [11].
The prognostic factors can be divided into four general groups: those associated with PS, tumor burden, systemic inflammatory markers, and treatment-related factors [20]. The PS is assessed using the Eastern Cooperative Oncology Group or KPS scales. The presence of constitutional symptoms, multiple sites of metastasis, level of LDH, and the presence of bone or liver metastasis have all been associated with a high tumor burden. Additionally, several proinflammatory response markers, including the erythrocyte sedimentation rate, C-reactive proteins, and neutrophilia, have also been identified as prognostic factors in cancer patients. The MSKCC, IMDC, and ARCC risk models use all these prognostic factors in different ways. In the case of the IMDC model, instead of the traditional elevated LDH level and nephrectomy status, it includes inflammation markers related to the overproduction of cytokines (neutrophilia and thrombocytosis). Moreover, in the current study, the IMDC model accurately predicted the prognosis of the non-clear cell RCC patients treated with temsirolimus as the first-line therapy. Kroeger et al. [18] also recently showed the reliability of the IMDC prognostic model for predicting OS and time to treatment failure in non-clear cell RCC patients. However, in their study, only 7% (n = 39) of the patients were treated with temsirolimus as their first-line therapy, and the non-clear RCC subtypes were not reported.
Interestingly, among the investigated scoring systems, the ARCC risk model produced the highest predictive values for the current patient population. The NCCN guidelines for RCC list six predictors of short survival when selecting patients for treatment with temsirolimus, and these poor prognostic features were defined in the Global ARCC trial [10,21]. In that trial, 80% of the patients (n = 502) had clear cell histology, and three or more poor prognostic factors were present in 94% (n = 589) of the patients. However, according to the MSKCC risk classification, only 74% (n = 462) of the patients were assigned to the poor risk group, indicating that some patients in the ARCC poor-risk group were considered intermediate by the MSKCC criteria. The unique differential feature of the ARCC model is the presence of organ metastasis. This factor of poor prognosis for patients with an increased tumor burden in RCC has already been described in a number of studies [14,15]. The current study also found multiple organ metastases in 79.7% of the patients. Thus, organ metastasis would appear to be a powerful tool for predicting the clinical outcomes for such patients.
Consistent with previous studies, the current study found an ORR of 8.2% and a DCR of 48.7%. In addition, the non-clear cell RCC patients had a shorter PFS and OS at 3 and 8 months, respectively [22]. The Global ARCC trial showed that temsirolimus significantly improved OS when compared with interferon-α in poor-risk metastatic RCC patients (10.9 months vs. 7.3 months). However, 80% of the patients had clear cell histology, and the histologic subtypes were not reported. While the current data have certain limitations, including its retrospective nature and a relatively small study sample, the advantages of the current study are as follows: (1) patient cohort with Korean homogenous ethnic identity, (2) patient cohort with non-clear cell histology, and (3) equivalent treatment application.
In conclusion, all three risk models reliably prognosticated the clinical outcomes of the non-clear cell RCC patients treated with temsirolimus as the first-line therapy. Furthermore, the ARCC risk model performed better than the other risk models in predicting survival. Further studies are needed to confirm these findings.
1. Non-clear cell renal cell carcinoma (RCC) patients had a shorter progression-free survival (PFS) and overall survival (OS) than clear cell RCC patients.
2. All three models reliably discriminated the risk groups to predict PFS and OS (p < 0.001) in metastatic non-clear cell RCC patients treated with temsirolimus.
3. The area under the ROC curve for progression was highest for the Advanced Renal Cell Carcinoma (0.777), followed by the International Metastatic RCC Database Consortium (0.756) and Memorial Sloan-Kettering Cancer Center (0.742) models.
Supplementary Materials
Supplementary Figure 1.
Comparison of receiver-operating characteristic (ROC) curves for (A) survival and (B) progression.
Figure 2.
Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival according to histologic subtypes (A: p = 0.018; B: p = 0.009).
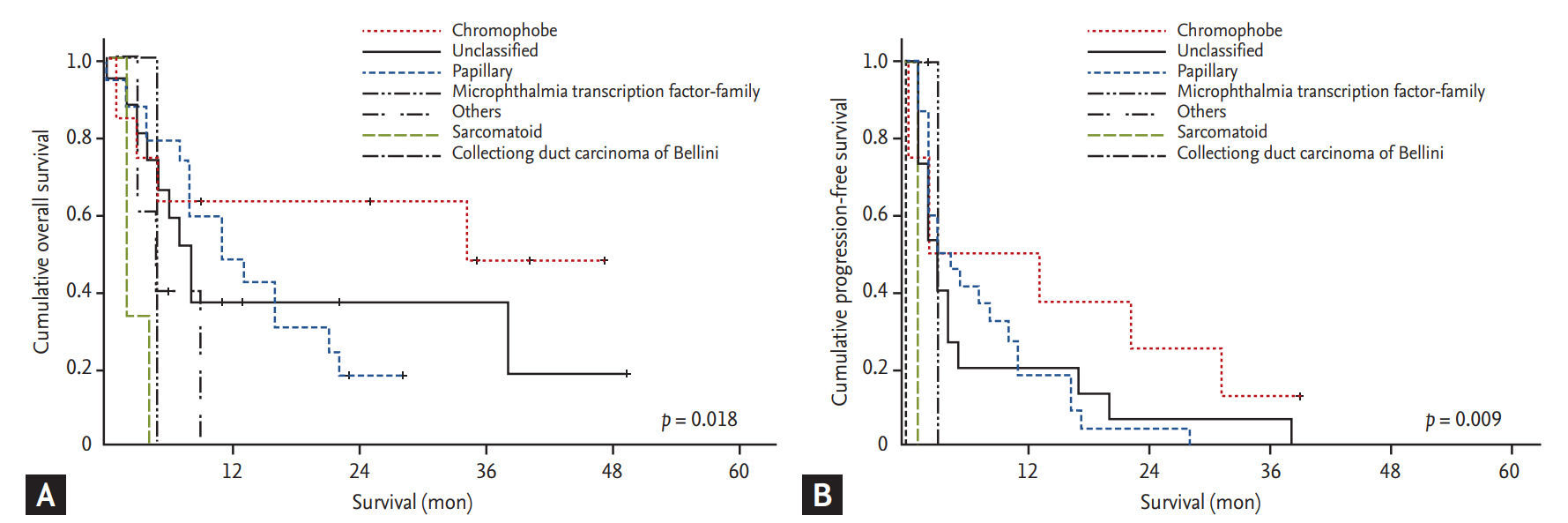
Figure 3.
Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival according to the Memorial Sloan-Kettering Cancer Center risk model (A: p < 0.001; B: p < 0.001).
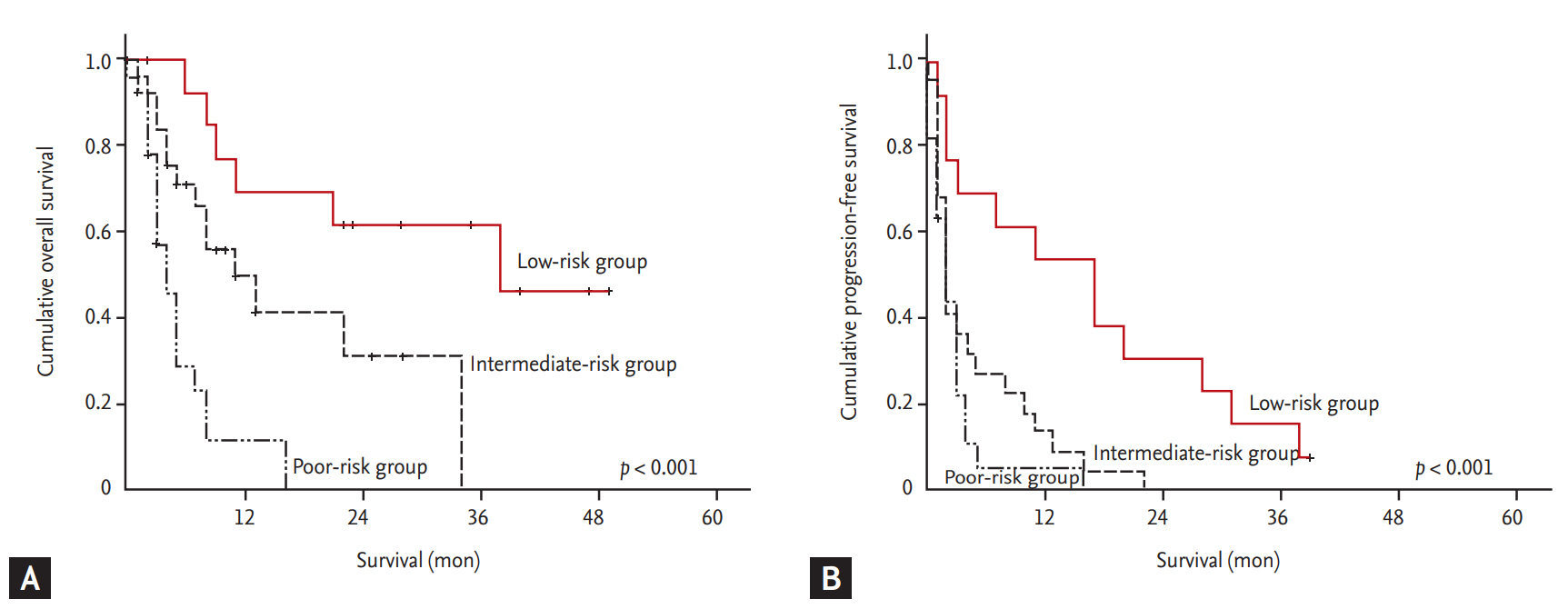
Figure 4.
Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival according to the International Metastatic RCC Database Consortium risk model (A: p < 0.001; B: p < 0.001).
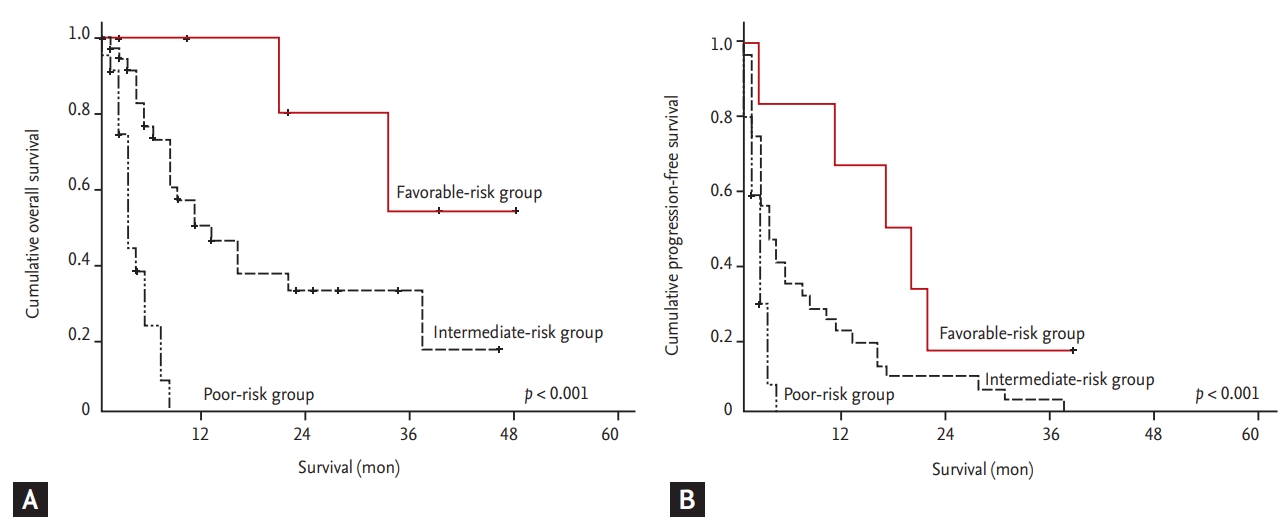
Figure 5.
Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival according to the Advanced Renal Cell Carcinoma risk model (A: p = 0.001; B: p = 0.004).

Table 1.
Patients and disease characteristics (n = 74)
Table 2.
Distribution of each scoring system
Table 3.
Best response of temsirolimus
| Response | No. (%) | Subtype |
|---|---|---|
| Best response | ||
| Complete response | 2 (3) | Chromophobe (1), unclassified (1) |
| Partial response | 5 (7.5) | Papillary (1), unclassified (4) |
| Stable disease | 27 (40.3) | Papillarya |
| Progressive disease | 26 (38.8) | Papillarya |
Table 4.
Area under the ROC curve for the three risk models
| Progression ROC area (95% CI) | Survival ROC area (95% CI) | |
|---|---|---|
| ARCC | 0.777 (0.653–0.873) | 0.734 (0.598–0.844) |
| IMDC | 0.756 (0.630–0.856) | 0.724 (0.587–0.836) |
| MSKCC | 0.742 (0.615–0.844) | 0.712 (0.574–0.826) |
Table 5.
Multivariate analysis of the prognostic factors for OS and PFS
REFERENCES
2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat 2017;49:306–312.




3. Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 Update on the classification of renal epithelial tumors in adults. Int J Urol 2009;16:432–443.


4. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol 2016;70:93–105.


6. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–1939.


7. Zeidan AM, Sekeres MA, Garcia-Manero G, et al. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia 2016;30:649–657.



8. Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256–4265.


9. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–134.


10. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–2281.


11. Li H, Samawi H, Heng DY. The use of prognostic factors in metastatic renal cell carcinoma. Urol Oncol 2015;33:509–516.


12. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–296.


13. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–2540.


14. Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23:832–841.


15. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799.


16. Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–148.



17. Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293–300.


18. Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013;119:2999–3006.



19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247.


20. Tang PA, Vickers MM, Heng DY. Clinical and molecular prognostic factors in renal cell carcinoma: what we know so far. Hematol Oncol Clin North Am 2011;25:871–891.


- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



