 |
 |
| Korean J Intern Med > Volume 34(2); 2019 > Article |
|
See editorial "The utility of ezetimibe therapy in nonalcoholic fatty liver disease" on page 284.
Abstract
Background/Aims
A number of clinical trials reported varying effects of cholesterol lowering agents in nonalcoholic fatty liver disease (NAFLD) patients. We, therefore, assessed the changes in hepatic steatosis and NAFLD activity score (NAS) after treatment with cholesterol lowering agents in NAFLD patients by metaanalysis.
Methods
The Cochrane Library, the MEDLINE, and the Embase databases were searched until May 2015, without any language restrictions, for randomized controlled trials (RCTs) and nonrandomized studies (NRSs). Additional references were obtained from review of bibliography of relevant articles. The quality of evidence was assessed using the grading of recommendations assessment, development and evaluation guidelines.
Results
Three RCTs (n = 98) and two NRSs (n = 101) met our study inclusion criteria (adult, NAFLD, liver biopsy). Liver biopsy was performed in all five studies, but only the three studies reported NAS. Ezetimibe significantly decreased NAS (standardized mean difference [SMD], –0.30; 95% confidence interval [CI], –0.57 to –0.03) but not hepatic steatosis in RCT (SMD, –0.1; 95% CI, –0.53 to 0.32), while the effect was significant for both NAS and intrahepatic content in NRSs (SMD, –3.0; 95% CI, –6.9 to 0.91).
Metabolic syndrome is a major risk factor for nonalcoholic fatty liver disease (NAFLD), with approximately half of all NAFLD patients also having hypercholesterolemia [1]. Current treatment for NAFLD consists largely of lifestyle modifications and treatment of comorbid conditions such as hyperlipidemia. Experimental studies in mice have shown that ezetimibe and statins not only reduce hepatic inflammation but also fibrosis [2]. Several studies also suggested that hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors may improve liver function tests and histology of NAFLD patients [3,4].
Results from both randomized control trials (RCTs) and non-RCT studies (NRSs) on the effects of cholesterol lowering agents have been difficult to interpret due to the variations in study designs, diagnostic criteria and types of cholesterol lowering agents used. For instance, a sub-study of the St. Francis heart study of 455 subjects showed that, 20 mg of atorvastatin combination with vitamins effectively reduced the odds of developing hepatic steatosis by 71% in healthy individuals as well as those with NAFLD [5]. Another study by Park et al. [6] which included 45 subjects showed ezetimibe as a promising agent for the treatment of NAFLD; however, this study did not have a control arm. In addition, most existing investigations were case control studies [7], and there are currently only four RCTs examining this important issue [4,8,9].
A recent Cochrane systematic review in 2013 identified only two RCTs with a total 205 participants, and neither study evaluated the histological response to statin therapy [7]. The authors concluded that there were insufficient evidence to either support or refute the use of statins in patients with NAFLD.
In the present study, we investigated the efficacy of cholesterol lowering agents in biopsy-proven NAFLD patients. Primary outcome was changes in hepatic steatosis, while the secondary outcomes were improvements in NAFLD activity score (NAS) as assessed by liver biopsy.
Two investigators independently searched MEDLINE (January 1, 1946 to May 30, 2015), Embase (January 1, 1947 to May 30, 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL; January 1, 1966 to May 30, 2015) without language or publication year restriction.
The following keywords, MeSH and free text were searched through MEDLINE: NAFLD, statin, and ezetimibe (Supplementary Table 1). Bibliographies of potentially relevant articles were manually reviewed to identify additional relevant studies. The identified articles were assessed individually for inclusion (Supplementary Table 2).
The studies were initially abstracted if they included the following keywords: NAFLD, statin, cholesterol lowering agent, ezetimibe. For inclusion, the studies were independently selected by two stages of screening using the Population Intervention Comparison Outcome framework [10]. Since the study objective was the histological effect with the lipid lowering agents, only those studies with liver biopsy results for diagnosis of NAFLD and post-treatment were included [11,12]. The required intervention included HMG-CoA reductase inhibitors or ezetimibe which can be administered at any dose for at least 6 months. The control group received no lipid lowering intervention or placebo, and there were no change of weight in all studies. The primary endpoint was improvement in hepatic steatosis while the secondary endpoint was improvement of NAS and safety.
Using a pre-defined data extraction form, two reviewers (H.Y.L. and D.W.J.) independently extracted data from each study. Any disagreement was independently reviewed by a third reviewer (H.J.K.). The following variables were extracted from the selected studies: (1) hepatic steatosis as evaluated by liver biopsy and/or quantitative fat measurement by magnetic resonance imaging (MRI); (2) NAS measurement before and after therapeutic intervention. All outcomes were assessed by differences between treated and control groups. The results were expressed as mean and standard deviations.
Two reviewers (H.Y.J. and D.W.J.) independently assessed the methodological qualities of included studies. The study quality was evaluated using the risk of bias by Cochrane for RCTs (Supplementary Fig. 1) and Newcastle Ottawa scale for NRSs (Supplementary Table 2) [13]. Any unresolved disagreements between reviewers were resolved by the third author (H.J.K.). Publication bias was not assessable due to the small numbers of studies.
We analyzed continuous data using standardized mean difference to combine trials that measure the same outcome but utilized different methods. The primary outcome was change of hepatic steatosis by liver biopsy (and MRI quantification in one study). The histological grading in NAFLD, inflammation, and fibrosis was based on scoring systems by either Brunt et al. [14] or Kleiner [15]. Secondary outcome were changes in NAS.
To assess for heterogeneity, we estimated the proportion of between-study inconsistency due to true differences between studies (rather than differences due to random error or chance) using the I2 statistic, with values of 25%, 50%, and 75% considered low, moderate, and high, respectively. Outcomes were analyzed using random effects model and standardized mean difference (SMD) to assess changes in measurements made by different scales. All analyses were performed using RevMan version 5.2 (http://community.cochrane.org/). This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Revise and Meta-Analyses (PRISMA) statement.
Fig. 1 shows the details of literature research and selection process of the meta-analysis. The initial search strategy identified 857 articles. Of these, 667 publications were excluded after reviewing the title and abstract which indicated that they did not fulfill the selection criteria (Supplementary Table 3). For the remaining 39 articles [4,6-11,13-44], we performed full manuscript review and identified five relevant studies (three RCTs and two NRSs) to include in this meta-analysis.
Table 1 describes characteristics of the five included studies. The five studies comprised a total of 199 participants who received either statins (n = 47) or ezetimibe (n = 42) in the treatment group, and placebo (n = 97) or ursodeoxycholic acid (UDCA) (n = 13) in the control group. Ezetimibe was administered for 6 months and statins was administered from 6 months to 6 years [8,9]. Two studies were conducted in the USA, and one each in Japan, Sweden, and Romania. The study by Nelson et al. [4], Ekstedt et al. [28] and Georgescu and Georgescu [30] used statins, while the study by Loomba et al. [8] and Takeshita et al. [9] used ezetimibe as the lipid lowering agent. By inclusion criteria, liver biopsy was performed in all five studies, but magnetic resonance spectroscopy was also used in one study [8]. The control subjects received placebo except for those in the study by Georgescu and Georgescu [30] which used UDCA. In terms of race/ethnicity, three studies [4,28,30] included mostly or all Caucasian, while two studies [8,9] included mostly or all Asians. In both studies that used ezetimibe for 6 months, the baseline cholesterol level was within normal range (180 to 190 mg/dL). However, in the study by Georgescu and Georgescu [30] in which statin was used, the baseline cholesterol levels were high with mean level ranging 318 to 326 mg/dL. Loomba et al. [8] studied Caucasian subjects (baseline BMI, 33 to 34 kg/m2), while Takeshita et al. [9] studied East Asian subjects with lower BMI (baseline BMI, 28 to 31 kg/m2).
Among the three RCTs, the quality of two studies [8,9] was satisfactory, but one study [4] did not have random allocation sequence, optimal allocation concealment, and detailed data description. However, treated and control groups in all three RCTs were well-matched based on baseline characteristics with well-defined treatment response. In the two NRSs, subjects in the two groups were not well-matched since they were not randomized. The level of evidence and grade of recommendation for each outcome are summarized in Supplementary Table 2.
Cholesterol lowering agents did not significantly decreased the hepatic steatosis in NAFLD patients in the three RCTs (SMD, –0.10; 95% confidence interval [CI], –0.53 to 0.32) or in two NRSs (SMD, –3.00; 95% CI, –6.90 to 0.91) (Fig. 2).
Only two RCTs and one NRS reported the NAS data. Meta-analyzed result of the two RCT studies demonstrated a significant improvement of NAS (SMD, –0.30; 95% CI, –0.57 to –0.03). As shown in Fig. 3, pooled estimate of all three studies with available data also showed significant improvement. However, the mean reduction of NAS was modest: –1.0 in in Loomba et al. [8] and –0.65 in Takeshita et al. [9] but slightly higher in the case control study by Georgescu and Georgescu (mean, –1.7) [30].
There was no significant change in serum fasting glucose levels in the two RCTs (SMD, 0.20; 95% CI, –0.57 to 0.97) (Fig. 4) [8,9]. Data on glycated hemoglobin (HbA1c) changes were also only reported in these two RCTs and there was no significant change (SMD, 0.31; 95% CI, –0.15 to 0.76) (Fig. 5) [8,9].
Our meta-analysis showed that ezetimibe decreased NAS (SMD, –0.30; 95% CI, –0.57 to –0.03) without observable improvement in hepatic steatosis. A recent systematic review suggested that statin therapy may improve serum aminotransferase and ultrasound findings [7]. The fundamental differences between our meta-analysis and the previous systemic review were the quantitative methods used for assessment of hepatic steatosis. Our meta-analysis is based only on biopsy, and in one study also MRI-estimated proton density fat fraction (MRI-PDFF) to quantify hepatic fat contents. Contrary to the previous systemic review which included sonography studies for hepatic steatosis assessment, we excluded studies using the sonographic method because it is subjective and poorly quantifiable [11]. Computed tomography (CT) scan is also a less sensitive method to diagnose fatty liver [12], and is therefore a subjective method to estimate quantitative changes in intrahepatic fat content. Thus, we excluded sonographic and CT-based studies in our meta-analysis [32]. Recent data showed that MRI-PDFF has become the primary imaging modality to assess intrahepatic fat content due to their high correlation with liver histology [45], and its clinical use has also been approved by the Food and Drug Administration (FDA) in the USA MRI-PDFF has emerged as a reference standard to measure hepatic steatosis in the radiation zone and is used as the primary modality for endpoint measurement in several clinical trials [45].
Of the RCTs only the two studies which used ezetimibe included NAS data. These two studies showed an improvement in NAS. The study by Loomba et al. [8] used both MRI-PDFF and liver biopsy. In fact, the primary end point of the study was changes in hepatic steatosis as measured by MRI-PDFF, with paired liver biopsy performed in 77.8% of study subjects [8]. We analyzed this study as their biopsy results were well-matched with similar baseline NAS in both groups (5 points each) as well as similar proportion lost at follow-up in both groups (32% vs. 28%, respectively). Moreover, baseline characteristics were also similar in both groups with regards to cholesterol levels, sex and age distribution. These two studies showed a trend of improvement in alanine aminotransferase (ALT) but there was no significant improvement when all four studies with information on ALT were included in the meta-analysis (Supplementary Fig. 2).
Several recent studies have suggested that long-term use of statins could increase the risk of diabetes mellitus and raise serum glucose levels [46]. In this meta-analysis, we did not find any significant increase in fasting serum glucose or HbA1c levels following statin use for 6 months. Takeshita et al. [9] reported a significant increase in HbA1c following the treatment; however, there was no significant increase in HbA1c observed when improvement rate was analyzed. Moreover, statin administration was also not associated with liver toxicity (Supplementary Fig. 2).
This meta-analysis had several limitations. First, the number of included studies was small. As such, we had to pool two cholesterol lowering agents (statin and ezetimibe) together in our analysis. Even though both have lipid-lowering effects, their mechanism of action is different. Second, there is considerable heterogeneity in design and endpoints among the available studies. For example, only three of the five studies were RCTs. Third, there were considerable heterogeneity in the two NRSs. The study by Ekstedt et al. [28] found a greater improvement in hepatic steatosis but the mean baseline cholesterol level was higher in the ezetimibe intervention group than the control at, 264 mg/dL vs. 230 mg/dL (p = 0.04), respectively. In the study by Georgescu and Georgescu [30] the improvement of hepatic steatosis was also observed in the control group who were however treated with UDCA administration.
In summary, the current meta-analysis found that lipid lowering agents can improve NAS in subjects with NAFLD but effects in hepatic steatosis were not observed. Given the small number of available RCTs, as well as a small number of study subjects in interventional studies overall, further large scale RCTs are needed to effectively evaluate the effects of cholesterol-lowering agents in improving intrahepatic fat in NAFLD patients with high baseline cholesterol levels.
1. Ezetimibe decreased nonalcoholic fatty liver disease (NAFLD) activity score without improving hepatic steatosis.
2. Cholesterol lowering agents did not significantly decreased the hepatic steatosis in NAFLD patients.
3. There was no significant increase in glycated hemoglobin levels following statin use for 6 months.
Supplementary Materials
Supplementary Table 3.
Characteristics of excluded studies (ordered by study ID)
Supplementary Figure 1.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Supplementary Figure 2.
Forest plot for decrease of serum alanine aminotransferase (ALT). SD, standard deviation; IV, interval variable; CI, confidence interval; Std., standardized; RCT, randomized controlled trial; NRS, nonrandomized study.
Figure 1.
Preferred Reporting Items for Systematic Revise and Meta-Analyses (PRISMA) diagram of the literature search. CT, computed tomography.

Figure 2.
Forest plot for improving rate of intrahepatic fat. RCT, randomized controlled trials; SD, standard deviation; IV, interval variable; CI, confidence interval.
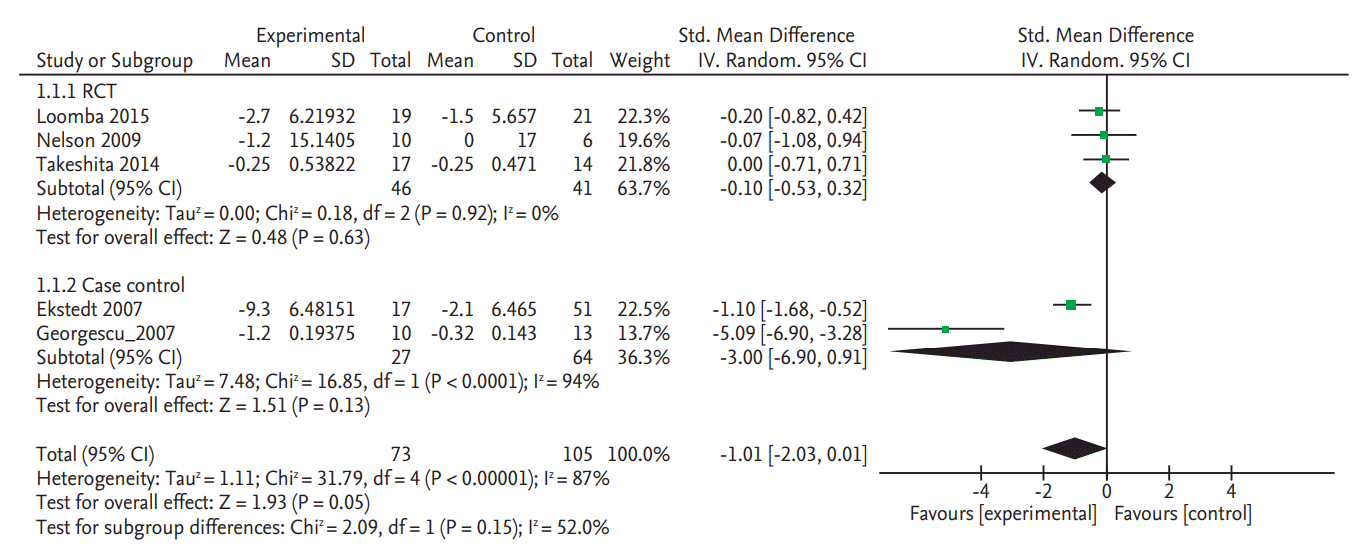
Figure 3.
Forest plot for improving rate of nonalcoholic fatty liver diseases activity score in randomized controlled trials (RCT). SD, standard deviation; IV, interval variable; CI, confidence interval.
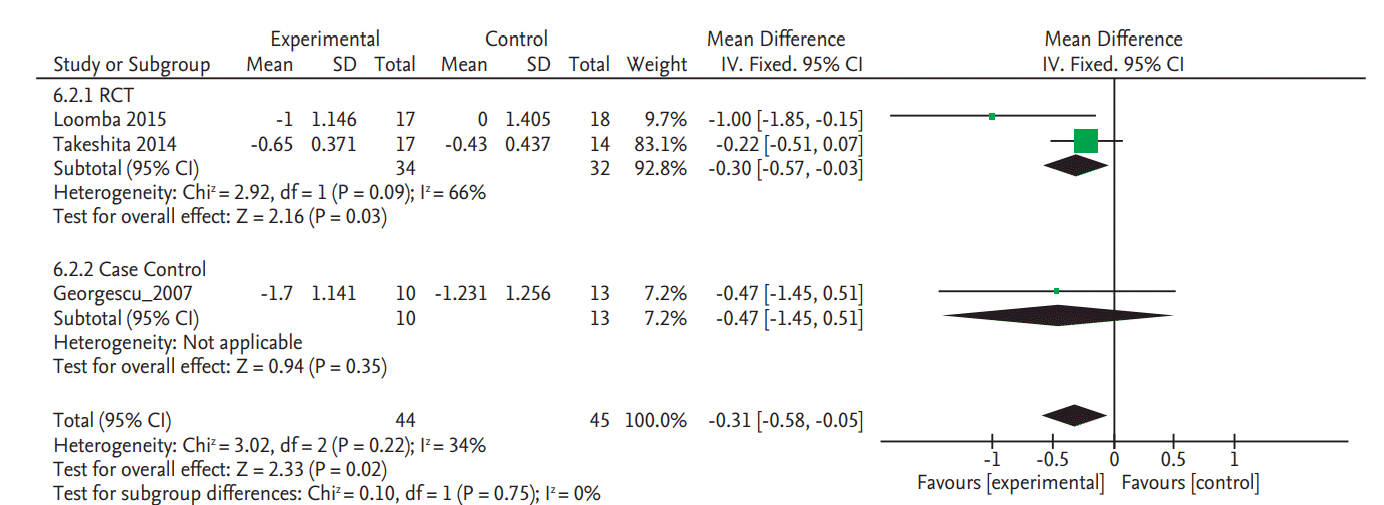
Figure 4.
Forest plot for decrease of serum fasting glucose. Std., standardized; SD, standard deviation; IV, interval variable; CI, confidence interval.

Figure 5.
Forest plot for decrease of serum glycated hemoglobin. Std., standardized; SD, standard deviation; IV, interval variable; CI, confidence interval.
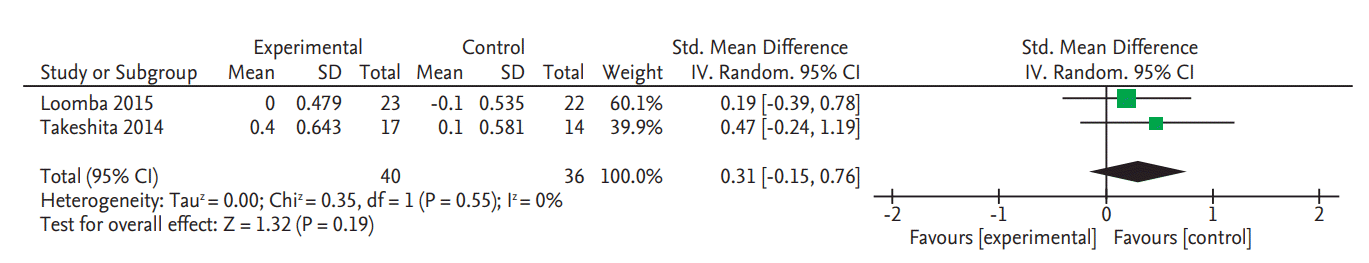
Table 1.
Study characteristics
| Variable | Nelson et al. (2009) [4] | Takeshita et al. (2014) [9] | Loomba et al. (2015) [8] | Georgescu et al. (2007) [30] | Ekstedt et al. (2007) [28] |
|---|---|---|---|---|---|
| Study design | RCT | RCT | RCT | Open label | Retroactive-prospective |
| Country | USA | Japan | USA | Romania | Sweden |
| Percentage with NASH | 100 | NR | 100 | 100 | 65 |
| Intervention | Simvastatin 40 mg | Ezetimibe 10 mg | Ezetimibe 10 mg | Atorvastatin 10 mg | Any statin |
| Treatment duration, mon | 12 | 6 | 6 | 18.7 | 73.2 |
| No. with repeat biopsya | 10 | 16 | 17 | 10 | 17 |
| Mean duration between biopsy | Baseline (within 6 mon) and 12 mon | Baseline and 6 mon | Baseline (within 6 mon) and 6 mon | Baseline (within 2 wk) and last visit | 13.8 ± 1.2 yr from first biopsy |
| Race/ethnicity | White, Hispanic, African | Asian | Caucasian | Caucasian | White |
| Sample size | 16 | 32 | 50 | 23 | 68 |
| Male sex | 11 | 20 | 19 | NR | 48 |
| Mean age, yr | 53 | 52.5 | 49.2 | 55 | 47.1 |
| Cholesterol, mg/dL | 208–231 | 170–199 | 170–182 | 318–326 | 230–264 |
| BMI, kg/m2, range | 34–37 | 28–31 | 33–34 | 35 | 27–30 |
| Percentage of diabetes | 28 | NR | 12 | NR | 80 |
| Improvement in histology, mean ± SD | |||||
| Steatosis (grade or %) | |||||
| Before | 25.0% ± 14.7% | 1.56 ± 0.18 | 2.00 ± 1.00 | 2.60 ± 0.27 | 20.4% ± 7.5% |
| After | 23.8% ± 21.2% | 1.31 ± 0.15 | 1.00 ± 1.00 | 1.40 ± 0.17 | 11.1% ± 8.9%b |
| p value | 0.8847 | 0.3000 | 0.2500 | 0.0001 | 0.001 |
| Lobular inflammation | |||||
| Before | NR | NR | 1.41 ± 0.49 | 1.80 ± 0.20 | 0 ± 0 |
| After | NR | NR | 1.65 ± 0.59 | 1.50 ± 0.17 | 0.12 ± 0.32 |
| Ballooning | |||||
| Before | NR | 0.69 ± 0.20 | 1.29 ± 0.57 | 1.10 ± 0.13 | 0.12 ± 0.32 |
| After | NR | 0.41 ± 0.15 | 1.00 ± 0.77 | 0.80 ± 0.10 | 0.41 ± 0.60 |
| Fibrosis | |||||
| Before | 1.25 ± 0.70 | NR | 1.35 ± 1.23 | 1.50 ± 0.17 | 0.88 ± 0.83 |
| After | 1.50 ± 0.90 | NR | 1.29 ± 1.28 | 1.30 ± 0.13 | 1.35 ± 1.33 |
| Mean NAS | |||||
| Before | NR | 3.71 ± 0.50 | 5.2 ± 2.00 | 6.70 ± 1.337 | NR |
| After | NR | 3.06 ± 0.45 | 4.0 ± 2.00c | 5.00 ± 1.563d | NR |
| p value | NR | 0.1850 | 0.2910 | 0.0176 | NR |
REFERENCES
1. Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci 2000;45:1929–1934.


2. Van Rooyen DM, Gan LT, Yeh MM, et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol 2013;59:144–152.


3. Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Statins and nonalcoholic fatty liver disease: a bright future? Expert Opin Investig Drugs 2013;22:1089–1093.


4. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009;43:990–994.


5. Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol 2011;106:71–77.



6. Park H, Shima T, Yamaguchi K, et al. Efficacy of longterm ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol 2011;46:101–107.


7. Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev 2013;(12):CD008623.


8. Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–1250.



9. Takeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia 2014;57:878–890.


10. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc 2006;2006:359–363.


11. Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:7392–7402.



12. Ma X, Holalkere NS, Kambadakone RA, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 2009;29:1253–1277.


13. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45.




14. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–2474.


15. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321.


16. Abel T, Feher J, Dinya E, Eldin MG, Kovacs A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with nonalcoholic fatty liver disease. Med Sci Monit 2009;15:MS6–MS11.

17. Abel T, Feher J, Dinya E, Gamal Eldin M, Kovacs A. Efficacy and safety of ezetimibe/simvastatin combination therapy in patients with type 2 diabetes and nonalcoholic fatty liver disease. Orv Hetil 2009;150:989–993.


18. Aggarwal V, Palmer CS, Yan KK, Lloyd AR, Zekry A. Statins and liver injury in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology 2009;50:789A.

19. Ahmed MH. Ezetimibe and recent clinical trials: a look on the bright side. Expert Opin Drug Saf 2010;9:511–514.


20. Arendt BM, Allard JP. Effect of atorvastatin, vitamin E and C on nonalcoholic fatty liver disease: is the combination required? Am J Gastroenterol 2011;106:78–80.



21. Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci 2016;61:1714–1720.



22. Bril F, Lomonaco R, Orsak B, et al. Safety of statin therapy in patients with prediabetes or T2DM and NASH: a longterm prospective study. Diabetes 2013;62(Suppl 1):A164.
23. Carnelutti A, Donnini D, Nadalutti G, et al. Effect of statin therapy vs diet in hypercholesterolemic patients affected by nonalcoholic steatohepatitis (NASH). Dig Liver Dis 2012;44(Suppl 1):S25–S26.

24. Chan DC, Watts GF, Gan SK, Ooi EM, Barrett PH. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care 2010;33:1134–1139.



25. De Keyser CE, Koehler EM, Schouten JN, Hofman A, Janssen HL, Stricker BH. Association between statin therapy and non-alcoholic fatty liver disease in a large population-based study. Pharmacoepidemiol Drug Saf 2013;22:373.
26. Drapkina OM, Ivashkin VT. Treatment of non-alcoholic fatty liver disease and dyslipidemia in patients with metabolic syndrome using simvastatin and ursodeoxycholic acid. Diab Vasc Dis Res 2011;8:56–57.
27. Drapkina OM, Korneeva ON, Ivashkin VT. Ademetionine and simvastatin in patients with nonalcoholic fatty liver disease and metabolic syndrome. Endocr Pract 2011;17:21A–22A.

28. Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol 2007;47:135–141.


29. Elsheikh E, Younoszai Z, Otgonsuren M, et al. Angiogenic growth factors associated with statins use in patients with nonalcoholic fatty liver disease (NAFLD) and coronary artery disease (CAD). Gastroenterology 2014;146(5 Suppl 1):S710.
30. Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH): are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis 2007;16:39–46.

31. Kargiotis K, Katsiki N, Athyros VG, et al. Effect of rosuvastatin on non-alcoholic steatohepatitis in patients with metabolic syndrome and hypercholesterolaemia: a preliminary report. Curr Vasc Pharmacol 2014;12:505–511.


32. Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 2003;17:713–718.



33. Koehler EM, De Keyser CE, Schouten JN, Hansen BE, Janssen HL, Stricker BH. Association between statin use and nonalcoholic fatty liver disease in a population-based study. Hepatology 2012;56 Suppl 1:595A–596A.
34. Maroni L, Castiglioni L, Marino F, et al. Achievement of lipid targets in non alcoholic fatty liver disease during statin treatment. J Hypertens 2010;28(Suppl A):e547.

35. Maroni L, Guasti L, Castiglioni L, et al. Lipid targets during statin treatment in dyslipidemic patients affected by nonalcoholic fatty liver disease. Am J Med Sci 2011;342:383–387.


36. Mihaila RG, Nedelcu L, Fratila O, Rezi EC, Domnariu C, Deac M. Effects of lovastatin and pentoxyphyllin in nonalcoholic steatohepatitis. Hepatogastroenterology 2009;56:1117–1121.

37. Oni E, Sinha P, Karim A, et al. Statin use is not associated with presence of and severity of non-alcoholic fatty liver disease. J Am Coll Cardiol 2013;61(10 Suppl S):E1427.

38. Patel A, Gawrieh S, Rizvi S, Xiang Q, Szabo A, Saeian K. Management strategies used for nonalcoholic fatty liver disease: survey of AASLD members. Gastroenterology 2009;136:A847.

39. Pireau L, Bailly S, Descamps OS. Does ezetimibe could correct hepatic steatosis? Acta Clin Belg 2013;68:463.
40. Reihner E, Rudling M, Stahlberg D, et al. Effect of pravastatin on hepatic cholesterol metabolism. Fortschr Med 1991;109:189–194.

41. Riley P, Sudarshi D, Johal M, et al. Weight loss, dietary advice and statin therapy in non-alcoholic fatty liver disease: a retrospective study. Int J Clin Pract 2008;62:374–381.


42. Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol 2011;12:80–85.


43. Skrypnyk IM, Dubrovins’ka TV. Optimization of longterm treatment with rosuvastatin of patients with myocardial infarction in combination with non-alcoholic steatohepatitis. Lik Sprava 2014;(5-6):113–121.
44. Zvenigorodskaja L, Cherkashova E, Samsonova N, Melnikova N. Evaluation of effectiveness in the hypolipidemic therapy of patients with atherogenic dislipoproteinemia and nonalcoholic fatty liver disease. Am J Hypertens 2009;22(Suppl 1):10.



- TOOLS
-
METRICS

- Related articles
-
The utility of ezetimibe therapy in nonalcoholic fatty liver disease2019 March;34(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


