 |
 |
| Korean J Intern Med > Volume 32(3); 2017 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
Acknowledgments
Supplementary Materials
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀTable┬Ā2.
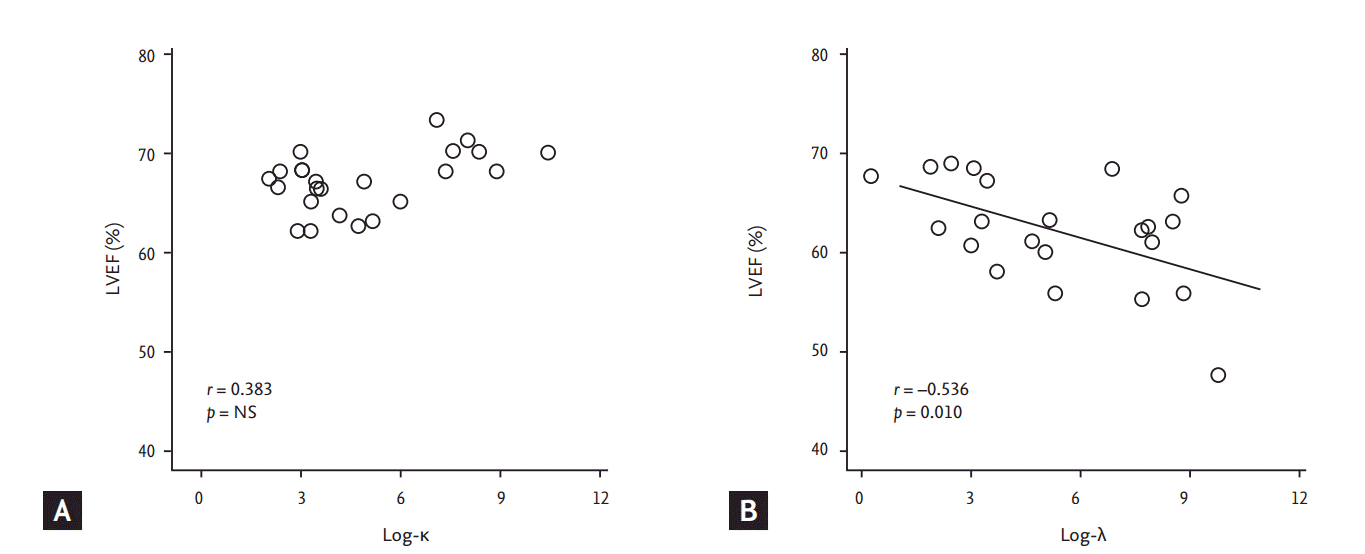
Figure┬Ā1.

Figure┬Ā2.
Table┬Ā1.
| Variable | Non-LCMM (n = 139) | LCMM (n = 45) | p value |
|---|---|---|---|
| Age, yr | 57.7 ┬▒ 9.3 | 56.3 ┬▒ 8.4 | 0.380 |
| Male sex | 69 (49.6) | 20 (22.5) | 0.544 |
| Body mass index, kg/m2 | 23.6 ┬▒ 2.9 | 23.8 ┬▒ 3.1 | 0.657 |
| Diabetes mellitus | 14 (10.1) | 5 (11.1) | 0.517 |
| Hypertension | 39 (28.1) | 14 (31.1) | 0.694 |
| Smoking | 10 (7.2) | 9 (20.0) | 0.019 |
| Induction chemotherapy regimens | 0.661 | ||
| ŌĆāBortezomib | 60 (43.1) | 24 (53.3) | |
| ŌĆāThalidomide | 37 (26.6) | 12 (26.6) | |
| ŌĆāBortezomib and thalidomide | 17 (12.2) | 4 (2.2) | |
| ŌĆāLenalidomide | 2 (1.4) | 0 | |
| ŌĆāHigh-dose dexamethasone | 23 (16.5) | 5 (11.1) | |
| Ig heavy chain isotype, G/A/D/M | 95/34/6/4 | - | |
| Ig free light chain isotype | 0.449 | ||
| ŌĆā╬║ Type | 80 (57.6) | 23 (51.1) | |
| ŌĆā╬╗ Type | 59 (42.4) | 22 (48.9) | |
| Durie-Salmon stagea | 0.055 | ||
| ŌĆāI | 3 (2.2) | 0 | |
| ŌĆāII | 18 (13.0) | 1 (2.2) | |
| ŌĆāIII | 117 (84.8) | 44 (97.8) | |
| ╬▓2-Microglobulin, mg/La | 3.5 (2.6ŌĆō6.4) | 5.3 (2.5ŌĆō11.2) | 0.076 |
| Albumin, g/dLa | 3.2 (2.8ŌĆō3.7) | 3.9 (3.5ŌĆō4.4) | < 0.0001 |
| Lactate dehydrogenase, U/La | 339 (264ŌĆō470) | 409 (342ŌĆō572) | 0.445 |
| Hemoglobin, g/dL | 11.1 (9.9ŌĆō11.8) | 10.4 (9.7ŌĆō11.3) | 0.161 |
| Hematocrit, % | 33.1 (29.7ŌĆō35.3) | 31.4 (29.9ŌĆō34.2) | 0.211 |
| Serum creatinine, g/dL | 0.7 (0.6ŌĆō0.9) | 0.9 (0.7ŌĆō1.8) | 0.032 |
| eGFRMDRD, mL/min/1.73 m2 | 98.6 (77.6ŌĆō120.6) | 69.9 (32.6ŌĆō110.9) | 0.021 |
| ESR, mm/hr | 32.0 (14.5ŌĆō61.0) | 19.0 (9.5ŌĆō34.2) | 0.015 |
| Total cholesterol, mg/dL | 170 (145ŌĆō202) | 159 (135ŌĆō189) | 0.404 |
| Triglyceride, mg/dL | 131 (85ŌĆō181) | 130 (101ŌĆō185) | 0.680 |
| Serum M protein, g/dL | 0.23 (0.01ŌĆō1.11) | - | |
| Serum FLC-╬║, mg/dL | 15.8 (9.0ŌĆō31.3) | 21.1 (10.3ŌĆō87.4) | 0.066 |
| Serum FLC-╬╗, mg/dL | 12.0 (7.3ŌĆō20.7) | 15.0 (8.8ŌĆō166.8) | 0.049 |
| Serum ╬║/╬╗ FLC-R | 1.25 (0.75ŌĆō3.58) | 1.20 (0.10ŌĆō5.03) | 0.511 |
Values are presented as mean ┬▒ SD, number (%), or median (interquartile range).
LCMM, light chain multiple myeloma; Ig, immunoglobulin; eGFRMDRD, estimated glomerular filtration rate using the Modification of Diet in Renal Disease; ESR, erythrocyte sedimentation rate; FLC, free light chain; FLC-R, free light chain ratio.
Table┬Ā2.
Values are presented as mean ┬▒ SD, number (%), or median (interquartile range).
LCMM, light chain multiple myeloma; LVEF, left ventricular ejection fraction; IVS, interventricular septal thickness; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVMi, left ventricular mass index; LAVi, left atrial volume index; DT, deceleration time; E/eŌĆÖ, peak early diastolic transmitral velocity to peak early diastolic mitral annular velocity; LVDD, left ventricular diastolic dysfunction; RVDd, right ventricular end-diastolic diameter; RVWT, right ventricular wall thickness; TAPSE, tricuspid annular plane systolic excursion; SPAP, systolic pulmonary artery pressure.
Table┬Ā3.
LCMM, light chain multiple myeloma; Log-╬║, log-transformed serum level of kappa; Log-╬╗, log-transformed serum level of lambda; LVEF, left ventricular ejection fraction; IVS, interventricular septal thickness; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVMi, left ventricular mass index; LAVi, left atrial volume index; DT, deceleration time; E/eŌĆÖ, peak early diastolic transmitral velocity to peak early diastolic mitral annular velocity; RVDd, right ventricular end-diastolic diameter; RVWT, right ventricular wall thickness; TAPSE, tricuspid annular plane systolic excursion; SPAP, systolic pulmonary artery pressure.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



