To the Editor,
Dasatinib (SPRYCEL1, Bristol-Myers Squibb, New York, NY, USA), a second-generation tyrosine kinase inhibitor (TKI), is widely used for patients with chronic myelogenous leukemia (CML) and Philadelphia (Ph) positive leukemia [1]. Dasatinib has 325-fold greater potency than imatinib. National Comprehensive Cancer Network and Korean Guidelines for CML treatment approve dasatinib as a first-line treatment along with imatinib and nilotinib. The adverse effects of dasatinib accompany myelosuppression, pleural effusion, diarrhea, and liver enzyme abnormalities. Gastrointestinal (GI) bleeding may occur during dasatinib treatment, but is generally mild and easily handled. However, we report the case of a 54-year-old male with CML in the accelerated phase (AP) with severe dasatinib-induced hemorrhagic colitis.
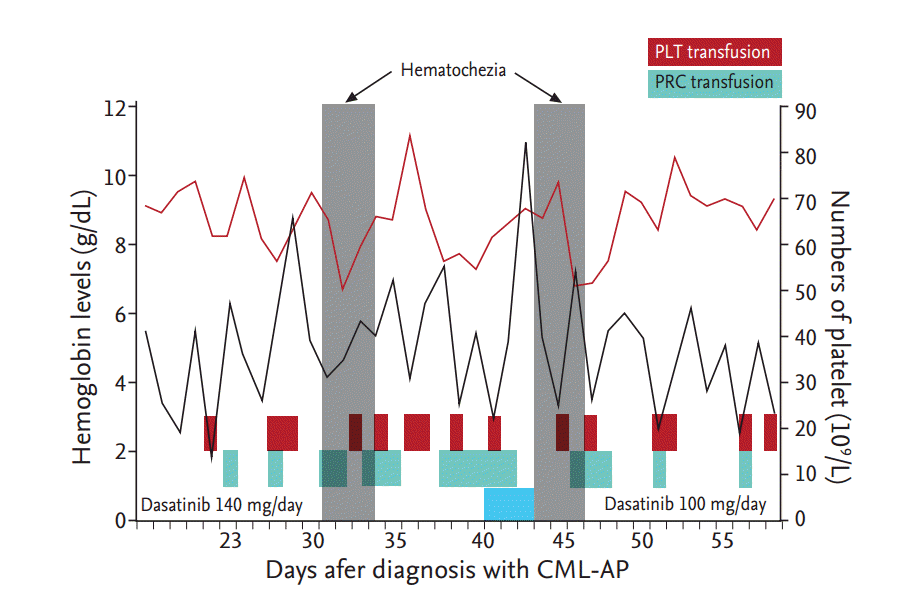
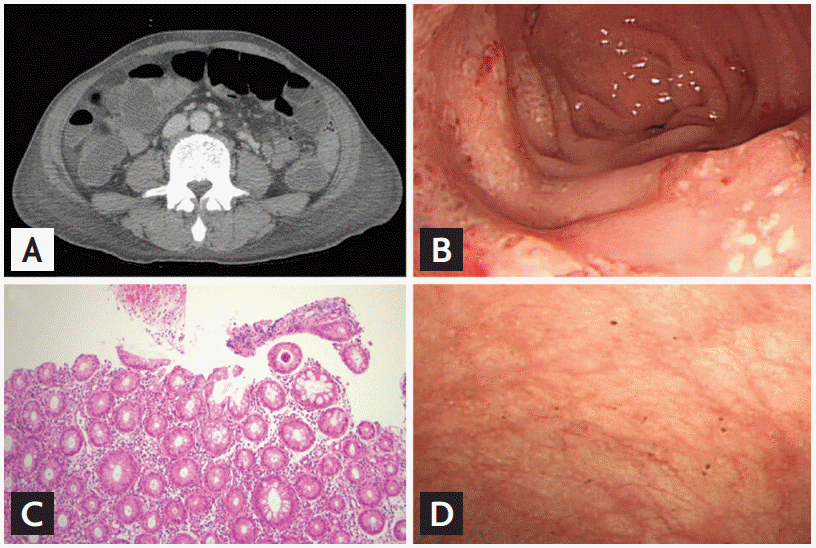
In 2011, he was diagnosed with CML in chronic phase (CP) with variant Ph chromosome translocations, t(9;10;22) (q34;q11.2;p13). He was initially treated with imatinib (400 mg daily). However, he did not achieve a major cytogenetic response even after 12 months of receiving imatinib, so dasatinib (100 mg daily) was used instead. Variant Ph chromosome translocations involving chromosome 10 represent a more aggressive marker in CML. The patient achieved a complete cytogenetic response after 3 months of dasatinib treatment (July 2012), and a major molecular response in international scale (BCR-ABL < 0.0001) after 12 months (April 2013). The patient continued to receive dasatinib and at the 36-month follow-up visit (April 2015), he developed anemia (9.7 g/dL) and mild thrombocytopenia (86 ├Ś 109/L) with blasts in the peripheral blood (12%). Bone marrow examination revealed increased blasts (16%) and variant Ph chromosome t(9;10;22) (q34;q11.2;p13) with an additional cytogenetic abnormality of inversion 3. He refused any further intensive treatments including bone marrow transplantation. The patient was given an elevated dose of dasatinib (140 mg daily), which rapidly decreased blasts from the peripheral blood. On day 30 post-treatment (June 2015), the patient developed hematochezia associated with a significant drop in hemoglobin levels (8.2 g/dL). Platelet count was 40 ├Ś 109/L on admission and coagulation profile was within the normal limit (international normalized ratio, 1.14; activated partial thromboplastin time 22.7 seconds). Hematochezia was still ongoing and the patient required the transfusion of 20 pints of packed red cells and 11 units of single donor platelets (Fig. 1). Platelet function testing with the PFA-100 assay (Siemens, Malvern, Australia) demonstrated impaired platelet aggregation; collagen/epinephrine closure time of 1,280 seconds (normal range, 82 to 290). Abdomen computed tomography scan revealed diffuse edematous bowel changes within the entire bowels (Fig. 2A). A total colonoscopy revealed no active bleeding, but there were multiple shallow ulcers with exudate and erythematous lesions on the mucosa involving the entire colon (Fig. 2B). The analyses of stool specimens were negative for parasites, clostridium difficile, and other pathogenic bacteria. The cytomegalovirus pp65 antigen was negative in his blood leukocytes. In the colonoscopic biopsy specimen, the crypt architecture was well-preserved and many lymphocytes infiltrated the lamina propria (Fig. 2C). Leukemic cells, viral inclusion or apoptotic bodies were not observed. An acid-fast stain using the colonoscopic biopsy specimen was also negative for mycobacterium infection. The patient was treated with broad-spectrum antibiotics, bowel rest and hydration. Dasatinib treatment was stopped to eliminate the possibility of dasatinib-induced hemorrhagic colitis. Dasatinib treatment was ceased and the hematochezia resolved without additional surgical or medical interventions. One week later, the PFA-100 assay was repeated with near restoration: collagen/epinephrine closure time of 154 seconds. The control colonoscopy was performed 10 days later and showed that the colonic mucosa was normal (Fig. 2D). We restarted dasatinib treatment with a reduced dosage (100 mg daily). On day 3 of treatment, the patient developed severe diarrhea with a large amount of intestinal hemorrhage (Fig. 1). Again, dasatinib treatment was stopped, and the hemorrhagic colitis drastically improved. We considered alternative TKIs like nilotinib, since it had no detectable inhibitory activities in platelet aggregations. However, the patientŌĆÖs condition aggravated and led to a discharge.
The introduction of TKIs in 2001, initially imatinib, has revolutionized the management of CML [1-3]. Imatinib resistance and/or intolerance, however, led to development and approval of more potent second generation TKIs, dasatinib and nilotinib, which induce faster and deeper molecular response rates and prominently used as CML front-line therapies [2,3]. However, a long-term follow-up on these agents demonstrates possible adverse effects. Of particular relevance for dasatinib is the potential for bleeding complications. Bleeding (mainly GI and epistaxis) has been reported in 40% of patients receiving dasatinib, grade 3 or 4 GI bleeding in 10% [1]. Here, we report the case of an adult patient diagnosed with CML-AP. The patient received dasatinib treatment. Dasatinib is a potent adenosine triphosphate-competitive inhibitor of BCR-ABL, cKIT, platelet-derived growth factor receptor, and SRC family kinases (SFKs). Dasatinib demonstrated high efficiency in patients with imatinib-resistant CML. SFKs are functionally involved in platelet activation and aggregation in immunoreceptor tyrosine based activation motif (ITAM)-mediated signalling pathways. SFK inhibition by dasatinib-impaired platelet signaling is initiated by collagen or Fc RIIA cross-linking, which require ITAM phosphorylation by SFKs [4]. Ninety-one patients with CML-CP receiving various first- and second-generation TKIs were evaluated with platelet aggregometry testing [3]. Dasatinib usage also resulted in a marked prolongation of closure time to collagen/epinephrine on the PFA-100 system, while the majority of patients receiving nilotinib had preserved platelet aggregation to all agonists. In this study, ADP-induced platelet aggregation was not significantly impaired by dasatinib [3]. Mice treated with increasing doses of dasatinib experienced significantly longer tail bleeding times than the control group in a dose-dependent manner [4]. This effect was evident after 4 hours; however, 24 hours after the drug administration, the effect of the drug diminishes and the bleeding times were decreased. By 48 hours, dasatinib was no longer detectable, demonstrating its reversible effect on hemostasis [4]. Furthermore, dasatinib can impact thrombopoiesis by promoting megakaryocyte differentiation within the bone marrow but impairing the ability of megakaryocytes to migrate and form proplatelets [2]. Therefore, thrombocytopenia is often observed in dasatinib therapy as a result of an impairment of megakaryocyte migration and maturation rather than a defect in megakaryocyte growth or an increase in platelet consumption. A growing body of experimental and clinical data demonstrated that dasatinib can impact platelet function in patients with CML, contributing to both impaired thrombopoiesis and more importantly, impaired adhesion and aggregation. Thus, the combination of thrombocytopenia and platelet dysfunction by dasatinib may be the cause of severe hemorrhage in our patient. Moreover, SFK is one of the key regulators in immune responses. SFK works to suppress activities of natural killer cells and T cells by inhibiting SFKs [5]. Therefore, dasatinib may cause acute colitis by decreasing immune tolerances to intestinal microflora through reducing the number of immunoregulatory cells and inhibiting the SFK signal transduction pathway. Such results can explain the inflammation with severe GI bleeding in the patient discussed in this case with no observed bleeding in other sites of body.
Physicians should keep in mind that unexpected bleeding might occur in patients with dasatinib treatment, and consider appropriate screening in patients receiving dasatinib. Discontinuation of dasatinib is essential in patients with bleeding complications. Also, it is not clear whether dasatinib can be re-administered to patients with bleeding complications because this patient developed re-bleeding after resuming of dasatinib. Thus, switching to the other TKI, such as nilotinib, should be considered in patients with advanced stage CML or Ph positive ALL receiving higher dosages of dasatinib (140 mg daily) than the recommended dosage for CML-CP (100 mg daily).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





