To the Editor,
Immune thrombocytopenia (ITP) is a disease caused by autoimmune destruction of platelets, and a disorder of megakaryopoiesis has also recently been recognized as an important mechanism involved in ITP. Current frontline therapeutic modalities for patients with ITP focus on intervening in the autoimmune destruction of platelets. In addition, the recent development of thrombopoietin (TPO) receptor agonists (RAs) offers a new approach for stimulating platelet production in the case of a failed frontline therapy or splenectomy. Romiplostim is an Fc-peptide fusion protein that stimulates the growth and maturation of megakaryocytes, thereby increasing platelet production, and is used to treat adults with refractory ITP via intravenous or subcutaneous injection. However, the available options for the treatment of refractory ITP following frontline or salvage therapies including steroids and the recommended dose of romiplostim remain very limited. In such cases, an escalated dose of romiplostim may be the only available approach. However, the issue of persistent romiplostim resistance and the use of high doses of romiplostim are raising concerns of adverse effects, such as bone marrow fibrosis or leukemogenesis [1].
Therefore, this brief report presents two cases of steroid-refractory ITP, in which, following the failure of treatment with 1 ╬╝g/kg of romiplostim, an excellent response was shown with the same dose of romiplostim in combination with prednisolone. For patients with refractory ITP who have already exhibited refractoriness to steroids and other treatments including azathioprine, romiplostim at a dose of 1 ╬╝g/kg every week is used as a salvage treatment. During the weekly romiplostim therapy, the two patients described in this report developed a dramatically decreased platelet count. We then added the prednisolone at a dose of 0.5 mg/kg to the weekly romiplostim therapy.
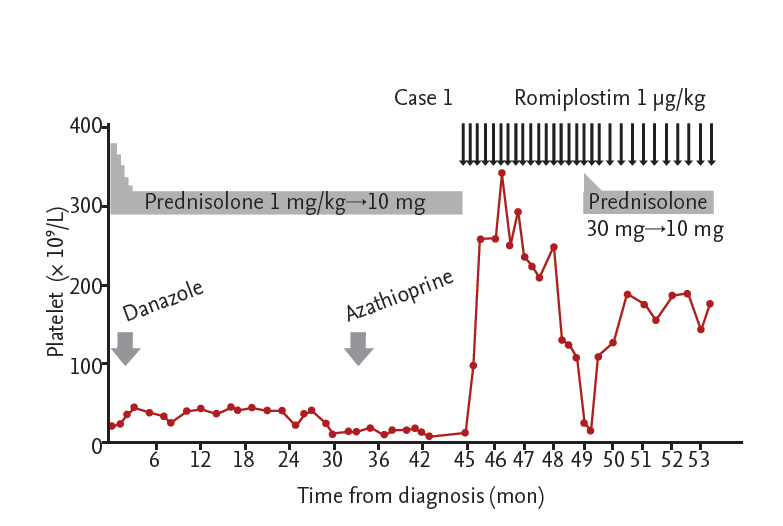
The first case describes a 67-year-old Korean woman who was diagnosed with ITP in October 2010. Her initial platelet count was 22 ├Ś 109/L. The patient showed refractoriness to prednisolone (1 mg/kg per day, then tapered). In December 2010, danazol (400 mg) was added to the tapered dose of prednisolone. A low dose of prednisolone has been maintained in patients due to the fear of a severe drop in the platelet count with the termination of prednisolone. While taking danazol and prednisolone, the patientŌĆÖs maximum platelet count only reached 45 ├Ś 109/L. In June 2013, azathioprine (100 mg) was added to prednisolone (10 mg per day) for 5 months, and this combination produced a platelet count of around 15 ├Ś 109/L. In July 2014, romiplostim (1 ╬╝g/kg per week) was tried without any other medication, and while the patient was receiving weekly injections of romiplostim (1 ╬╝g/kg for 15 weeks), her platelet count remained within the normal range. In October 2014, the patientŌĆÖs complete blood cell count suddenly revealed severe thrombocytopenia (platelet count 15 ├Ś 109/L), even though the hemoglobin values (12 to 16 g/dL) and white blood cell counts (4.8 to 10.8 ├Ś 109/L) remained normal. Despite continued romiplostim administration, the severe thrombocytopenia persisted for a month. As a salvage treatment, prednisolone (30 mg per day) was added while continuing the same dose of romiplostim (1 ╬╝g/kg per week), and the prednisolone was then tapered to 25 mg during the following week. The laboratory results showed a dramatic increase in the platelet count (109 ├Ś 109/L) after the patient had received the combination therapy for two weeks. The prednisolone was again tapered to 20 mg for 5 days and then to 15 mg for 5 days while maintaining the same dose of romiplostim (1 ╬╝g/kg) every 10 days. At the point at which the patient was taking 10 mg of prednisolone daily with the same dose of romiplostim (1 ╬╝g/kg every 10 days), her platelet count reached 126 ├Ś 109/L. Thereafter, the platelet count remained within the normal range with the combined administration of romiplostim and a low dose of prednisolone (Fig. 1).
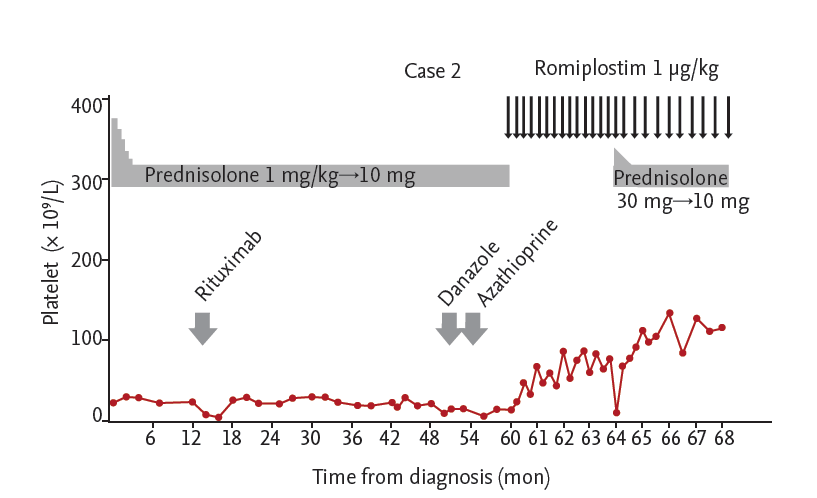
In the second case, a 52-year-old Korean woman presented with a severe bleeding tendency in July 2009. Her initial platelet count was 22 ├Ś 109/L. The patient did not show any increase in the platelet count upon the administration of prednisolone (1 mg/kg per day, then tapered). In October 2010, the platelet count was still low at around 20 ├Ś 109/L, even with rituximab therapy, resulting in a diagnosis of steroid-refractory ITP. The addition of danazol (400 mg) also failed in November 2013, and there was no response when azathioprine (100 mg/day) was added to the low dose of prednisolone for 3 months. In July 2014, the platelet count showed a modest increase to 40 ├Ś 109/L when the patient was taking only romiplostim at a dose of 1 ╬╝g/kg every week. However, after 14 weeks of romiplostim therapy, the platelet count suddenly dropped to 10 ├Ś 109/L even with continued weekly romiplostim. In November 2014, prednisolone was added at a dose of 30 mg for 5 days and 25 mg for the next 5 days while continuing the same dose of romiplostim (1 ╬╝g/kg per week), and the platelet count increased to 77 ├Ś 109/L. The romiplostim schedule was then switched to an injection every 10 days with a tapered dose of prednisolone at 20 mg for 5 days and 15 mg for the next 5 days. At the point at which the patient was taking 10 mg of prednisolone daily with the same dose of romiplostim (1 ╬╝g/kg every 10 days), her platelet count reached 142 ├Ś 109/L. Thereafter, the platelet count remained within the normal range with the combined administration of romiplostim and a low dose of prednisolone (Fig. 2).
Concurrent treatment with TPO-RAs and immunosuppressants has been previously reported with positive results in cases of chronic refractory ITP patients [2,3]. Combination therapy may enhance the effect of TPO-RAs by modifying the immunological environment. On the other hand, the cases described here provide evidence of a new synergistic efficacy with the combination of romiplostim and a steroid, despite confirmed refractoriness to both romiplostim and the steroid. The mechanism of the synergistic efficacy of this combination is not yet known. Immunosuppressive therapy reduces platelet destruction, whereas romiplostim encourages platelet production [1]. Thus, one possible explanation for this synergistic action is as follows: the exposure to romiplostim derived from exogenous proteins may stimulate the production of neutralizing antibodies, which can reduce the effectiveness of the drug. According to Amgen Co.ŌĆÖs report, pre-existing anti-romiplostim antibodies were identified in 8% (43/537) of patients treated with romiplostim in clinical trials, and anti-TPO antibodies were identified in 5% (29/537) [4]. Thus, the combination with a steroid may enhance the stability of romiplostim by blocking the action of the romiplostim antibodies. Since the production of romiplostim antibodies has been reported as negligible and romiplostim has no amino acid sequence homology to endogenous TPO, exposure to romiplostim has not been expected to elicit immunogenic responses against endogenous TPO, but the emergence of romiplostim antibodies is still an important concern [1]. Unlike human erythropoietin and human growth hormone, TPO has two different affinity cytokine receptor homology (CRH) domains in c-Mpl. Thus, while the romiplostim resistance to the immunologic roles of antipeptide immunoglobulin Gs reactive to the cytotoxic T lymphocyte epitope is not fully understood, it may be related to an interaction between the TPO CRH domains in c-Mpl [5]. Data from several studies showed that the recovery of the platelet count depended on the romiplostim dosage [1]. However, no drug accumulation was observed in the plasma and no linear correlation was found between the serum drug concentration and the dosage. Therefore, in this setting, it is speculated that even an escalated dose of romiplostim will be limited in its ability to reverse romiplostim resistance. Furthermore, the possible adverse effects of romiplostim, such as thrombosis, bone marrow fibrosis, and malignant hematopoiesis, should not be ignored [1].
Thus, the recovery of megakaryopoiesis using a TPO-RA may have had a paradoxical effect on the endogenous TPO level and then possibly the thrombocytopenia. In this situation, adding a steroid may have reversed the paradoxical thrombocytopenic status based on the synergistic action of the combination therapy.
In conclusion, the current cases demonstrate that the addition of a steroid without an increase in the dose of romiplostim can play a rescue role for patients with refractory or relapsed ITP. The immunosuppressive treatment seemingly acts in cooperation with romiplostim against romiplostim antibodies, thereby favoring platelet production and survival, and prolonging the effect of romiplostim. Yet, whatever the mechanism, the additional use of a steroid would seem to play an important role in restoring the efficacy of romiplostim for those patients who previously showed resistance to romiplostim.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





