 |
 |
| Korean J Intern Med > Volume 31(5); 2016 > Article |
|
Abstract
Background/Aims:
Cytomegalovirus (CMV) surveillance and preemptive therapy is a widely-used strategy for preventing CMV disease in transplant recipients. However, there are limited data on the incidence and patterns of CMV disease during the preemptive period. Thus, we investigated the incidence and pattern of tissue-invasive CMV disease in CMV seropositive kidney transplantation (KT) and hematopoietic stem cell transplantation (HCT) recipients during preemptive therapy.
Methods:
We prospectively identified patients with tissue-invasive CMV disease among 664 KT (90%) and 496 HCT (96%) recipients who were D+/R+ (both donor and recipient seropositive) during a 4-year period.
Results:
The incidence rates of CMV disease were 4.1/100 person-years (4%, 27/664) in KT recipients and 5.0/100 person-years (4%, 21/496) in HCT recipients. Twenty-six (96%) of the KT recipients with CMV disease had gastrointestinal CMV, whereas 17 (81%) of the HCT recipients had gastrointestinal CMV and 4 (19%) had CMV retinitis. Thus, CMV retinitis was more common among HCT recipients (p = 0.03). All 27 KT recipients with CMV disease suffered abrupt onset of CMV disease before or during preemptive therapy; 10 (48%) of the 21 HCT recipients with CMV disease were also classified in this way but the other 11 (52%) were classified as CMV disease following successful ganciclovir preemptive therapy (p < 0.001).
Cytomegalovirus (CMV) infection is a major infectious complication following kidney transplantation (KT) [1] and hematopoietic stem cell transplantation (HCT) [2]. Since the introduction in clinical practice of CMV antigenemia and polymerase chain reaction (PCR) as surveillance tools, a preemptive therapy has become the widely used preventive method in both KT [3] and HCT [4]. This is true also in Korea where most individuals are CMV seropositive [5]. Despite the effectiveness of the preemptive therapy, occasional cases of tissue-invasive CMV disease occur [6-9]. However, data on the incidence and patterns of CMV disease during the preemptive period are limited. In particular, there have been few studies comparing CMV disease in solid organ transplant recipients and in HCT recipients. Comparing to KT recipients with CMV disease, a lower CMV antigenemia or viremia has been reported in HCT recipients [10]. This difference may be come from intense immunosuppression, prolonged and frequent neutropenia, and post-transplant immune-reconstitution in HCT [11-14]. If the different patterns of CMV disease are shown between KT and HCT, it can be assumed that these factors affect the pathogenesis of development of CMV diseases. In addition, analyzing the patterns of CMV diseases in ‘real-world’ can provide evidence for designing preventive strategies appropriate for the different types of transplantation. Therefore, we investigated the incidence and pattern of tissue-invasive CMV disease in CMV seropositive KT and HCT recipients during preemptive therapy.
All adult recipients undergoing KT between May 2009 and May 2012 and allogeneic HCT between January 2009 and June 2013, were enrolled at a 2,700-bed tertiary-care hospital in Seoul, South Korea. A preemptive therapy has been used in seropositive KT recipients (R+) since May 2009, and in all HCT recipients regardless of donor or recipient CMV serostatus since January 2009. Universal oral ganciclovir (GCV) prophylaxis for 3 months without CMV antigen monitoring was also employed in KT recipients where the donor was seropositive and the recipient seronegative (D+R–). Infectious disease specialist, hematologics, and transplant surgeons have well cooperated and the standardization of practice has been established in both KT and HCT, respectively. Therefore, the KT cohort among various types of solid organ transplantation was chosen for the comparison with the HCT cohort. To select a homogenous population, only D+R+ KT and HCT recipients were included in the final analysis. We prospectively identified patients with tissue-invasive CMV disease, and retrospectively reviewed the medical records of all the enrolled subjects. The study was approved by the Institutional Review Board of Asan Medical Center and the requirement for informed consent was waived because of the retrospective nature of the study.
The immunosuppressive regimens comprised induction therapy with either basiliximab or anti-thymocyte globulin, followed by triple maintenance therapy with tacrolimus or cyclosporine, mycophenolate mofetil or azathioprine, and corticosteroids. Recipients who were ABO-incompatible or positive for human leukocyte antigen cross-matching received rituximab (500 mg) for 7 to 10 days before KT. The ABO-incompatible recipients underwent plasmapheresis until the anti-A or anti-B titer was < 1:8. CMV infection was routinely monitored by the CMV pp65 antigenemia assay (Light Diagnostic CMV pp65 Antigenemia, Chemicon International Inc., Temecula, CA, USA) at 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 weeks after KT [15]. It took just 1 to 2 days for doctors to get to know a CMV antigenemia result in hospitalized situation, but it depended on revisit schedule in out-patient department. If serum CMV antigen was ≥ 50/200,000 cells, intravenous GCV (10 mg/kg per day) or oral vangalciclovir (1,800 mg per day) was administered until CMV antigen was reported negative [15]. This cut-off level was determined by our own previous retrospective analysis [16]. Recipients who received antiviral agents continued to be tested for CMV antigenemia throughout the first year.
During the study period, surveillance testing for CMV pp65 antigenemia was carried out weekly from day 21 to day 100 post-HCT, then monthly until 1 year after HCT. Recipients who received antiviral agents in the first 100 days continued to be tested for CMV antigenemia throughout the first year. CMV antigenemia of ≥ 5/200,000 cells in high risk recipients and ≥ 20/200,000 cells in low-risk recipients were indications for antiviral agents. This protocol was designed according to the previous study using a low level CMV antigenemia cut-off level in HCT [17]. Myeloablative conditioning regimens included busulfan plus cyclophosphamide or busulfan plus fludarabine. The reduced-intensity conditioning regimen included busulfan, fludarabine, and antithymocyte globulin. Cyclosporine and methotrexate were given for graft-versus-host disease (GVHD) prophylaxis. Patients receiving antithymoglobulin in the preparative regimen, those with grade 3 to 4 acute GVHD, and those receiving more than 0.5 mg/kg methylprednisolone were classified as high risk. At the discretion of the attending hematologist, conventional dose GCV (10 mg/kg per day) or low dose (5 mg/kg per day) GCV was used as preemptive regimen until recipients were negative for CMV antigenemia, as described in the previous study [17].
CMV infection was considered to be present when positive cells were detected in the CMV antigenemia assay, or when CMV disease was diagnosed, irrespective of CMV antigenemia. CMV gastrointestinal disease was defined as symptoms and signs of upper or lower gastrointestinal dysfunction and tissue biopsy containing CMV inclusions, or positive immunohistochemical staining [18]. CMV pneumonitis was defined as symptoms of dyspnea and interstitial infiltrations on chest radiography, confirmed by bronchoalveolar lavage cytology or culture [19]. CMV retinitis was diagnosed based on documentation of typical lesions by an ophthalmologist. Patients with CMV disease were stratified into two groups: group A, those having abrupt onset of CMV disease before or during antiviral therapy; group B, those having CMV disease following successful antiviral therapy. Breakthrough CMV disease was defined as the occurrence of CMV disease more than 7 days after GCV or valganciclovir preemptive therapy in patients who did not have any symptoms and signs at the time of the start of antiviral agents.
All statistical analysis was carried out with SPSS version 21.0 (IBM Co., Armonk, NY, USA). The categorical variables were compared by Fisher exact tests or Pearson chi-square tests, as appropriate, and the continuous variables were compared by the Mann-Whitney U test or the Student t test. Incidence rates were compared using the Poisson distribution. All tests were two-tailed and differences were considered significant at p < 0.05.
During the study period, a total of 741 KT and 518 HCT recipients were enrolled. Of these, 664 KT (90%) and 496 HCT recipients (96%) who were D+/R+ (both donor and recipient seropositive) were included in the final analysis. In the KT cohort, 395 recipients (60%) gave positive CMV antigenemia results with ≥ 1/200,000 cells: 77 (12%) with 1 to 4/200,000 cells, 240 (36%) with 5 to 49/200,000, and 78 (12%) with ≥ 50/200,000 cells. Among the latter 78 recipients, 66 (10%) received GCV therapy according to the predefined threshold (see METHODS) and 12 (2%) underwent negative conversion without antiviral agents. These 12 patients did not develop any CMV-related problems. In the HCT cohort, 345 patients (70%) had positive CMV antigenemia results with ≥ 1/200,000 cells, and 202 (41%) received GCV therapy according to the predefined threshold (see METHODS).
Of the enrolled recipients, 27 KT recipients (4%, incidence rate 4.1/100 person-years; 95% confidence interval [CI], 2.7 to 6.0) and 21 HCT recipients (4%, incidence rate 5.0/100 person-years; 95% CI, 3.1 to 7.7) developed tissue-invasive CMV disease (p = 0.49). Median absolute neutrophil count (ANC) at the time of CMV tissue was lower in HCT recipients (2,332 µ/L; interquartile range [IQR], 2,645 to 5,333) than that in KT recipients (3,771 µ/L; IQR, 2,645 to 5,333; p = 0.012). But, only one recipient had less than 1,000 µ/L ANC in both groups, respectively (Table 1). Median post-transplant days at the onset of CMV disease in the KT recipients and HCT recipients were 51 (IQR, 35 to 88) and 60 (IQR, 40 to 115; p = 0.30) (Table 1). Four KT recipients (15%) and eight HCT recipients (38%) had their first episode of CMV disease > 100 days post-transplantation (p = 0.10). Two KT recipients (7%) and none of the HCT recipients had their first episode > 180 days post-transplantation (p = 0.50). Of 27 KT recipients with CMV disease, 26 (96%) had gastrointestinal disease, whereas, of the 21 HCT recipients with CMV disease, 17 (81%) had gastrointestinal CMV diseases and four (19%) had CMV retinitis (Figs. 1 and 2). Thus, CMV retinitis was more frequent in the HCT recipients (p = 0.03) (Table 1).
As shown in Figs. 1 and 2, all 27 KT recipients with CMV disease were classified as group A (abrupt onset of CMV disease before or during antiviral therapy), while 10 (48%) of the 21 HCT recipients with CMV disease were classified as group A and the other 11 (52%) as group B (successful antiviral therapy followed by CMV disease) (p < 0.001). Median post-HCT days of the first episodes of CMV disease in group A and group B were 40 (IQR, 27 to 51) and 110 (IQR, 76 to 140), respectively (p < 0.001). Numbers of episodes needing antiviral therapy before the first onset of CMV disease were: once in 73% (8/11), twice in 18% (2/11, HCTB1 and HCTB5), and three times in 9% (1/11, HCTB3) of group B patients. Among the KT recipients with CMV diseases, none had recurrent CMV disease.
The numbers of patients suffering an abrupt onset of the episode of CMV disease without a preceding positive result for CMV antigenemia (circles in Figs. 1 and 2) were four (15%) in the KT cohort and eight (38%) in the HCT cohort (p = 0.10). In the KT cohort, seven (26%) had nonsignificant levels (less than 50) of CMV antigenemia previously (triangles in Fig. 1): three (11%) with 1 to 4/200,000 cells (KTA1, KTA26, and KTA27), four (14%) with 5 to 49/200,000 cells (KTA11, KTA17, KTA21, and KTA23). Remaining 16 (59%) had significant levels (≥ 50/200,000 cells) of preceding CMV antigenemia (rectangles in Fig. 1). In the HCT cohort, six (29%) had non-significant levels (less than 5) of CMV antigenemia previously (triangles in Fig. 2), and the remaining seven (33%) had significant levels (5 or more) of CMV antigenemia (rectangles in Fig. 2). Four KT recipients (15%) (KTA2, KTA16, KTA25, and KTA27) and five (24%) of the HCT recipients (HCTA8, HCTA9, HCTB3, HCTB8, and HCTB10) had negative results for CMV antigenemia at the time of diagnosis of CMV disease (p = 0.48). Breakthrough CMV disease occurred in three KT (11%) and two HCT (10%) recipients (p > 0.99): KTA12, KTA15, and KTA20 in the KT cohort and HCTA2 and HCTA10 in the HCT cohort.
Surveillance and preemptive therapy is the widely used strategy for preventing CMV disease in both KT [3] and HCT [4], because universal prophylaxis appears to be associated with higher cost, adverse effects and late CMV infection [1,6,7,20,21]. However, some KT [6-8] and HCT [9] patients on preemptive therapy suffer from CMV disease. We found a similar incidence rate of CMV disease in KT (4.1/100 person-years) and HCT (5.0/100 person-years) patients during preemptive therapy. Separate previous studies of KT recipients or HCT recipients have reported rates of CMV disease after KT and HCT of 0.8% to 9.0% [7,8,22] and 5.8% [9], respectively. Our findings on the rates of CMV disease in KT and HCT recipients are in line with previous studies.
Although the incidence of CMV disease is similar between KT and HCT recipients during preemptive therapy, the different patterns of CMV disease development were found in our study: a higher number of patients with CMV disease after successful antiviral therapy and CMV retinitis in HCT recipients. KT and HCT recipients had different conditions such as immunosuppression intensity, neutropenia, and post-transplant immune-reconstitution. These differences may alter the patterns of CMV diseases development [11-14]. Therefore, this type of comparison may provide new insight into the CMV pathogenesis. In addition, the first step in reducing the development of CMV disease is to understand its pattern during preemptive therapy. This will provide valuable information for designing an individualized preventive therapy according to the type of transplant. However, data on the pattern of development of CMV disease in this small minority of patients is limited. To our knowledge, this is the first study to compare directly the incidence and patterns of CMV disease in KT recipients to that in HCT recipients during similar periods and in the same medical center.
The first way for CMV disease to develop is as a breakthrough disease despite antiviral therapy. In our study, only three (11%) of the 27 KT recipients and two (10%) of the 21 HCT recipients followed this pattern. Possible reasons for breakthrough infection could be the development of GCV-resistant CMV or CMV disease having been missed before the start of the GCV therapy. Unfortunately, we did not investigate the presence of GCV-resistant mutants in these five patients, so it is impossible to demonstrate it. The second way for CMV disease to develop is when the preceding CMV antigenemia below the threshold for antiviral therapy. In the present study, 26% (7/27) of patients receiving KT and 29% (6/21) of those receiving HCT displayed this pattern. A possible method for preventing this type of disease would be to lower the antigenemia threshold for antiviral therapy. However, this could result in unnecessary exposure to antiviral drug toxicity. If the level of CMV antigenemia for preemptive therapy in the KT cohort had been lowered to ≥ 5/200,000 cells in our study, four cases (patients with preceding CMV antigenemia from 5 to 49/200,000 cells) might have been prevented, but an additional 240 recipients would have received GCV treatment. Also, if the level of CMV antigenemia for preemptive therapy had been lowered to ≥ 1/200,000 cells in the HCT cohort, 143 more recipients would have received GCV treatment and this might have prevented at most 3 cases (triangles in group A, Fig. 2A). A cost-effectiveness study is needed to determine the appropriate threshold for antiviral therapy. The final way for the disease to develop is abruptly without any preceding CMV antigenemia. In our study, this pattern of development of CMV disease was observed in 15% (4/27) of the patients receiving KT and 38% (8/21) of those receiving HCT. Further studies are needed to determine whether more sensitive surveillance methods such as quantitative PCR for CMV would be useful for preventing CMV disease in this type of patient.
As shown in Fig. 2, CMV disease following successful antiviral therapy occurred in about a half of the HCT patients with CMV disease. Interestingly, none of KT recipients developed CMV disease following successful antiviral therapy. It seems that in some HCT patients even antiviral therapy cannot prevent the development of CMV disease but only delays its onset (group B in Fig. 1). One possible explanation is the absence of a CMV-specific T-cell response in these patients, or delayed reconstitution of a response. Li et al. [23] reported that antiviral therapy delayed the establishment of a protective CMV-specific T-cell response, and it has been shown that the absence of such a response, due to immunosuppressive therapy in patients with acute GVHD, is associated with the acquisition of CMV disease [24]. Therefore, delayed reconstitution of a CMV-specific T-cell response may cause recurrent CMV infection and the eventual development of CMV disease [25]. This hypothesis should be tested by comparing CMV-specific T-cell responses in HCT recipients with and without recurrent CMV infection and/or CMV disease. Based on ongoing studies on immunopathogenesis of CMV diseases, detection of CMV-specific T-cell responses may be used in the CMV prophylaxis strategy.
In the present study, some patients gave negative results for CMV antigenemia at the time of diagnosis of CMV disease (11% in KT and 24% in HCT). Green et al. [9] reported that 79% (33/41) of patients in whom CMV disease developed in the first 100 post-HSCT days did not give positive results for CMV antigenemia in a screening test. We previously reported that, in HCT or solid organ transplant recipients, the sensitivity of CMV antigenemia of ≥ 5/200,000 cells for the diagnosis of CMV gastrointestinal disease and pneumonia was 71% [18] and the sensitivity of CMV antigenemia of ≥ 1/200,000 cells for the diagnosis of CMV pneumonia was 69% [19]. These findings might be explained by a low sensitivity of CMV antigenemia as a tool for diagnosing tissue-invasive CMV disease. Another possible explanation is that reactivation of CMV in CMV seropositive recipients may not always lead to CMV viremia in tissue-invasive CMV disease. In fact, the sensitivity of CMV PCR for diagnosing CMV gastrointestinal disease was significantly lower in CMV seropositive solid organ transplant recipients (73%) than in CMV seronegative ones (100%) [26]. Since in Korea, seropositivity for CMV is very high among adults [5] (95% of the patients in this study were of CMV donor-positive and CMV recipient-positive serostatus), this partially explains the low or negative results for CMV antigenemia at the time of diagnosis of CMV disease in one third of the patients with CMV disease.
It is worth mentioning that gastrointestinal CMV disease was the most common end organ disease of CMV infection in both KT and HCT recipients, but CMV retinitis occurred fairly frequently in the HCT recipients. The reason for this is not clear. Previous studies of CMV end organ disease in HIV patients revealed that CMV retinitis is the most common end organ disease [27,28]. Thus gastrointestinal CMV disease is relatively uncommon in HIV patients compared with transplant recipients. We suppose that this interesting phenomenon may be explained by different immune responses in the different hosts. For example, we found that HIV patients with CMV retinitis did not have any CMV-specific T-cell response despite high blood CMV viremia (unpublished data), which suggests that overflow CMV replication can result in CNS CMV disease. Moreover, we detected some CMV-specific T-cell responses of CMV gastrointestinal disease in KT recipients at the time of diagnosis of CMV disease, which suggests a better-controlled form of CMV disease. Recently, commercial assays including enzyme linked immunosorbent assay-based assays (i.e., QuantiFERON-CMV, QIAGEN, Venlo, Netherlands) and the Enzyme-Linked ImmunoSpot-based assay (T-track-CMV) have become available, so further studies of CMV-specific T-cell responses in different hosts with CMV disease might answer this question.
There are some limitations to the present study. First, the incidence of CMV disease may have been underestimated because episodes of uncertain diagnosis, such as CMV syndrome and CMV pneumonia without results of CMV culture of bronchoalveolar lavage, were not categorized as tissue-invasive CMV disease. CMV gastrointestinal disease may be missed from HCT recipients, because they could not tolerate gastroscopy or colonoscopy study. Second, due to the specific circumstances of the Korean national medical insurance system, PCR was not routinely used for surveillance. Comparing to CMV PCR, CMV antigenemia had a lower sensitivity [10]. However, in the light of previous results [1,9,29], it can be assumed that the use of PCR as a surveillance tool would not yield very different results. Third, a lower cut-off level for antiviral therapy in HCT recipients may bias the comparison of CMV incidence between KT and HCT recipients. However, in the present study, we tried to reflect a ‘real-world’ situation and verify the current CMV antigenemia cut-off level. Fourth, delayed GCV treatment in patients with CMV antigenemia could eventually result in CMV disease. Indeed, 36 (55%) of the 66 KT recipients with CMV antigenemia received GCV therapy 1 week after the detection of CMV antigenemia, because it took at least 1 to 2 weeks for them to revisit the outpatient clinic. However, there was no significant difference in the delay in starting antiviral therapy between patients suffering and not suffering CMV disease (data not shown). Fifth, longer surveillance period in HCT cohort might bias towards that more group B patients be found in HCT. But, it seems to have limited influence on the result, since intense surveillance period was only for 100 post-transplant days in HCT and KT recipients receiving antiviral therapy also monitored during 1 year. Finally, due to the small number of patients with CMV disease, it was not possible to compare the clinical characteristics of the patients with different patterns of CMV disease.
In summary, the incidence of CMV disease was about 4% in both KT and HCT recipients during preemptive therapy. However, some patients receiving preemptive therapy might not be fully evaluated for the diagnosis of CMV diseases and the incidence may be underestimated. CMV retinitis was more frequent in HCT recipients than KT recipients and CMV disease developed as a relapsed infection especially in the HCT recipients receiving prior antiviral therapy. The different patterns of tissue-invasive CMV diseases in KT and HCT recipients implies that preemptive therapy protocol should be adjusted according to the type of transplant. Further studies are needed to reduce the number of failed cases during preemptive therapy.
1. The incidence rates of cytomegalovirus (CMV) disease were 4.1/100 person-years (4%, 27/664) in kidney transplantation (KT) recipients and 5.0/100 person-years (4%, 21/496) in hematopoietic stem cell transplantation (HCT) recipients during preemptive therapy.
2. CMV disease developed as a relapsed infection especially in the HCT recipients receiving prior antiviral therapy.
3. The different patterns of tissue-invasive CMV diseases in KT and HCT recipients implies that preemptive therapy protocol should be adjusted according to the type of transplant.
Acknowledgments
This study was supported by a grant of the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HI15C1763).
Figure 1.
Pattern of tissue-invasive cytomegalovirus (CMV) disease in kidney (KT) recipients during preemptive therapy. A plain figure without borderline lying on the patient number means a type of preceding CMV antigenemia: a circle, no preceding CMV antigenemia; a triangle, preceding nonsignificant CMV antigenemia (< 50/200,000 cells); a rectangle, preceding significant CMV antigenemia (≥ 50/200,000 cells). A plain figure with a thick borderline means time of diagnosis and type of tissue-invasive CMV disease. Numbers written below plain figures of KTA1 and KTA2 means time of diagnosis of tissue-invasive CMV diseases developing more than 180 days post-KT.
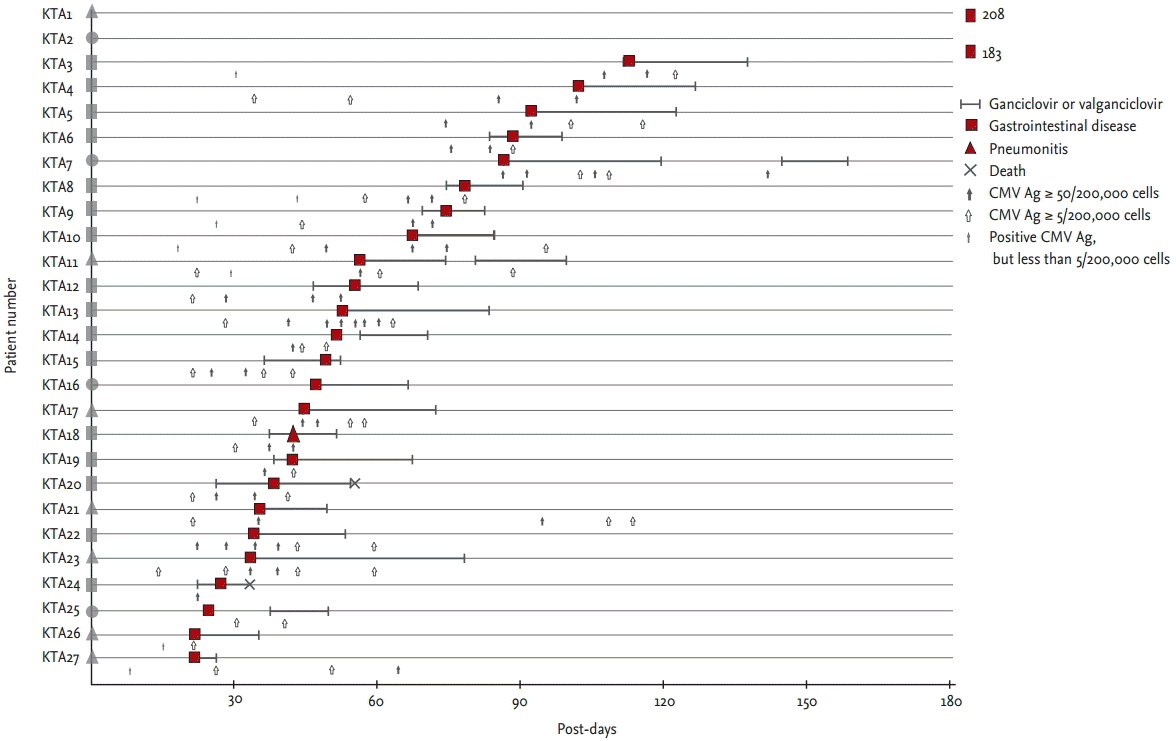
Figure 2.
Pattern of tissue-invasive cytomegalovirus (CMV) disease in allogeneic hematopoietic stem cell transplant (HCT) recipients during preemptive therapy. (A) It shows the pattern of 10 HCT recipients (48%) with CMV disease were classified as group A (abrupt onset of CMV disease before or during antiviral therapy), while (B) shows the other 11 (52%) as group B (successful antiviral therapy followed by CMV disease). Median post-HCT days of the first episodes of CMV disease in group A and group B were 40 (interquartile range [IQR], 27 to 51) and 110 (IQR, 76 to 140), respectively (p < 0.001). A plain figure without borderline lying on the patient number means a type of preceding CMV antigenemia: a circle, no preceding CMV antigenemia; a triangle, preceding nonsignificant CMV antigenemia (< 5/200,000 cells); a rectangle, preceding significant CMV antigenemia (≥ 5/200,000 cells). A plain figure with a thick borderline means time of diagnosis and type of tissue-invasive CMV disease. A number written below plain figure of HCTA8 means time of diagnosis of tissue-invasive CMV diseases developing more than 180 days post-HCT.
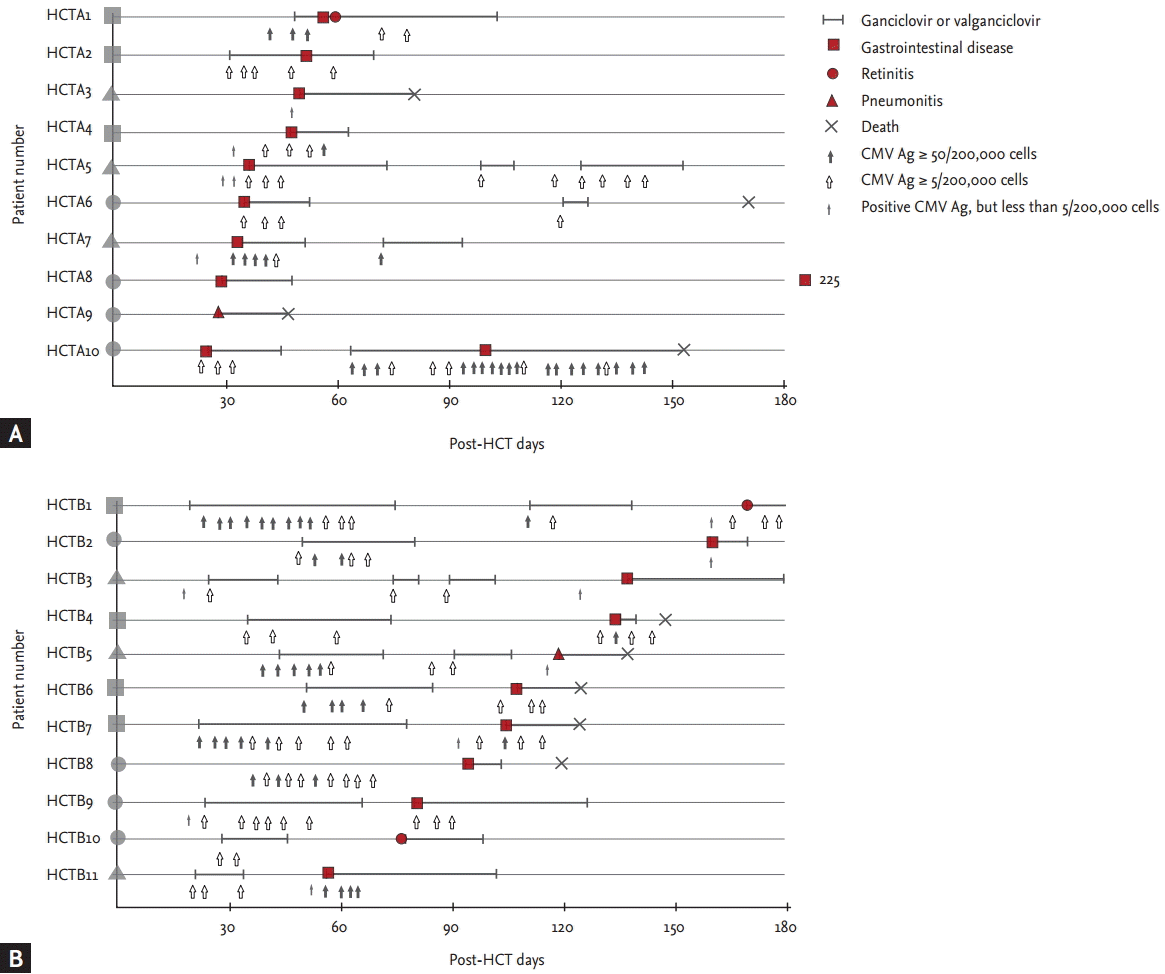
Table 1.
Comparison of the characteristics of tissue-invasive CMV disease in KT and allogeneic HCT recipients during preemptive therapy
| Characteristic | KT (n = 27) | HCT (n = 21) | p valuea |
|---|---|---|---|
| Age, yr, median (IQR) | 55 (45–58) | 50 (36–54) | 0.07 |
| Male sex | 14 (52) | 13 (62) | 0.56 |
| Incidence, /100 person-year (95% CI) | 4.1 (2.7–6.0) | 5.0 (3.1–7.7) | 0.49 |
| Absolute neutrophil count at the onset time, median (IQR), µg/L | 3,771 (2,645–5,333) | 2,332 (1,411–4,560) | 0.01 |
| Absolute neutrophil count < 1,000 | 1 (4) | 1 (5) | > 0.99 |
| Time of initial episode from transplantation, median (IQR), day | 51 (35–88) | 60 (40–115) | 0.30 |
| More than 100 days post-transplantation | 4 (15) | 8 (38) | 0.10 |
| More than 180 days post-transplantation | 2 (7) | 0 | 0.50 |
| Peak level of CMV antigenemia, median (IQR), /200,000 cells | 343 (98–1,078) | 81 (35–1,301) | 0.13 |
| Preceding CMV antigenemia, median (IQR), /200,000 cells | 190 (15–385) | 6 (3–11) | < 0.001 |
| With significant preceding CMV antigenemiab | 16 (59) | 7 (33) | 0.008 |
| Without preceding CMV antigenemia | 4 (15) | 8 (38) | 0.10 |
| Without concurrent CMV antigenemia | 4 (15) | 5 (24) | 0.48 |
| Type of infection | |||
| Gastrointestinal disease | 26 (96) | 17 (81) | 0.15 |
| Retinitis | 0 | 4 (19)c | 0.03 |
| Pneumonia | 1 (4) | 2 (10) | 0.57 |
| Recurrent CMV infection after CMV disease | 2 (7) | 5 (24) | 0.22 |
| Recurrent CMV disease | 0 | 2 (10) | 0.19 |
| CMV disease following successful ganciclovir therapy (group B) | 0 | 11 (52) | < 0.001 |
| Breakthrough CMV diseased | 3 (11) | 2 (10) | > 0.99 |
| Overall mortality | 2 (7) | 8 (38) | 0.01 |
Values are presented as number (%) of patients unless otherwise indicated.
CMV, cytomegalovirus; KT, kidney transplant; HCT, hematopoietic stem cell transplant; IQR, interquartile range; CI, confidence interval.
a Fisher exact test for categorical variables, Mann-Whitney U test for continuous variables, and the Poisson distribution for incidence rates were used to compare.
REFERENCES
1. Razonable RR, Humar A, AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:93–106.


2. Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant 2003;9:543–558.


3. Le Page AK, Jager MM, Kotton CN, Simoons-Smit A, Rawlinson WD. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation 2013;95:1455–1460.


4. Avery RK, Adal KA, Longworth DL, Bolwell BJ. A survey of allogeneic bone marrow transplant programs in the United States regarding cytomegalovirus prophylaxis and pre-emptive therapy. Bone Marrow Transplant 2000;26:763–767.


5. Han JS, Lee SJ, Park WK, Ko YW, Kim HO, Lee SY. A survey on the cytomegalovirus antibodies in blood donors and the diseased. Korean J Blood Transfus 1990;1:21–34.
6. Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 2006;6:2134–2143.


7. Reischig T, Jindra P, Hes O, Svecova M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 2008;8:69–77.


8. Jung GO, Kim SJ, Choi GS, et al. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc 2010;42:804–810.


9. Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:1687–1699.



10. Mori T, Mori S, Kanda Y, et al. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2004;33:431–434.


11. Burns LJ, Miller W, Kandaswamy C, et al. Randomized clinical trial of ganciclovir vs acyclovir for prevention of cytomegalovirus antigenemia after allogeneic transplantation. Bone Marrow Transplant 2002;30:945–951.


12. Schetelig J, Oswald O, Steuer N, et al. Cytomegalovirus infections in allogeneic stem cell recipients after reduced-intensity or myeloablative conditioning assessed by quantitative PCR and pp65-antigenemia. Bone Marrow Transplant 2003;32:695–701.


13. Ozdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 2002;100:3690–3697.


14. Hambach L, Stadler M, Dammann E, Ganser A, Hertenstein B. Increased risk of complicated CMV infection with the use of mycophenolate mofetil in allogeneic stem cell transplantation. Bone Marrow Transplant 2002;29:903–906.


15. Ko GB, Kim T, Kim SH, et al. Increased incidence of herpes zoster in the setting of cytomegalovirus preemptive therapy after kidney transplantation. Transpl Infect Dis 2013;15:416–423.


16. Kim T, Sung H, Park KT, et al. Clinical usefulness of human cytomegalovirus antigenemia assay after kidney transplantation. Infect Chemother 2009;41:72–77.

17. Park SY, Lee SO, Choi SH, et al. Efficacy and safety of low-dose ganciclovir preemptive therapy in allogeneic haematopoietic stem cell transplant recipients compared with conventional-dose ganciclovir: a prospective observational study. J Antimicrob Chemother 2012;67:1486–1492.


18. Jang EY, Park SY, Lee EJ, et al. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis 2009;48:e121–e124.


19. Moon SM, Sung H, Kim MN, et al. Diagnostic yield of the cytomegalovirus (CMV) antigenemia assay and clinical features in solid organ transplant recipients and hematopoietic stem cell transplant recipients with CMV pneumonia. Transpl Infect Dis 2012;14:192–197.


20. Couzi L, Helou S, Bachelet T, et al. Preemptive therapy versus valgancyclovir prophylaxis in cytomegalovirus-positive kidney transplant recipients receiving antithymocyte globulin induction. Transplant Proc 2012;44:2809–2813.


21. Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother 2013;45:260–271.



22. Greiner M, Cusini A, Ruesch M, et al. A stringent preemptive protocol reduces cytomegalovirus disease in the first 6 months after kidney transplantation. Infection 2012;40:669–675.


23. Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood 1994;83:1971–1979.


24. Tey SK, Kennedy GA, Cromer D, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One 2013;8:e74744.



25. Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis 2012;55:1678–1689.


26. Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013;57:1550–1559.



27. Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS): clinical findings, diagnosis, and treatment. Ann Intern Med 1988;108:585–594.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



