 |
 |
| Korean J Intern Med > Volume 29(1); 2014 > Article |
|
Abstract
Background/Aims
We investigated the agreement between the QuantiFERON-TB Gold (QFT-Gold) test and the tuberculin skin test (TST) in the diagnosis of latent tuberculosis infection in patients with rheumatoid arthritis (RA), compared with healthy controls, in Korea.
Methods
We recruited 64 patients with RA and 79 healthy controls at two university hospitals in South Korea. The participants underwent both the QFT-Gold test and the TST simultaneously between August 2006 and February 2009. All patients were diagnosed using the classification criteria for RA revised in 1987 by the American College of Rheumatology. Bacillus Calmette-Gu├®rin vaccination status and current medications were evaluated, and disease activities were assessed using the Disease Activity Score in 28 joints. Eleven patients with RA produced indeterminate QFT-Gold test results and were thus excluded from the kappa analysis.
Results
Based on an induration of 10 mm in diameter as the TST cutoff value, the QFT-Gold test and TST demonstrated 75.0% agreement (╬║ = 0.23) in patients with RA and 75.9% agreement (╬║ = 0.19) in healthy controls. Among the 56 patients with RA who had negative TST results, 11 patients (17.2%) also yielded indeterminate QFT-Gold results.
The accurate diagnosis of latent tuberculosis infection (LTBI) has become an important issue in the field of rheumatology. Patients with rheumatoid arthritis (RA) are at increased risk of developing serious infections, which, in Korea, commonly manifests as tuberculosis (TB).
In the detection and prophylactic treatment of LTBI, diagnostic tools for LTBI are used to decide whether antitumor necrosis factor-╬▒ (anti-TNF-╬▒) agents should be administered [1]. There is an intermediate prevalence of active pulmonary TB in Korea, and Bacillus Calmette-Gu├®rin (BCG) vaccination is mandatory [2]. The accuracy of the tuberculin skin test (TST) for the diagnosis of LTBI is controversial, especially with regard to TB burden, BCG vaccination, and immune status [3,4]. Despite these limitations, the TST is used routinely in hospital clinical practice to screen for LTBI.
Two new interferon (IFN)-╬│ release assays may offer improved specificity and sensitivity over the TST for the diagnosis of LTBI [5,6]. One of these, the QuantiFERON-TB Gold (QFT-Gold), showed encouraging results in low-risk BCG-vaccinated subjects [7] and patients with active TB [6]. QFT-Gold overcomes some of the shortcomings of the TST, such as the need for return visits, reader variability, variable specificity, and cross reactivity with BCG vaccination and nontuberculous mycobacterial infections [8-10]. Although these tests are valuable in screening for LTBI, the diagnostic accuracy varies according to the patient population. Furthermore, several studies in Korea have suggested a discordance between the QFT-Gold test and the TST [11-13]. One study showed a poor correlation between the TST and the IFN-╬│ assay in healthy Korean volunteers [11]. Two other studies reported poor agreement between TST and IFN-╬│ assay results in Korean patients with RA [12,13].
However, it remains unclear whether the QFT-Gold test can replace the TST in patients with RA or in the general population of Korea. We need clear practice guidelines for the diagnosis and treatment of LTBI to reduce the TB infection rates in patients with rheumatologic diseases who receive anti-TNF-╬▒ agents. However, there are no reported estimates of the concordance between the two diagnostic tools for LTBI in patients with RA or healthy volunteers in Korea.
Thus, in this study, we investigated the agreement between the QFT-Gold test and the TST in patients with RA and healthy controls to assess differences between the laboratory tests and the testers. We also estimated the clinical significance of indeterminate QFT-Gold results.
We enrolled 64 patients with RA at two university hospitals in South Korea between August 2006 and February 2009. Patients with RA were diagnosed according to the RA criteria set in 1987 by the American College of Rheumatology. None of the patients had been administered an anti-TNF-╬▒ agent. Among the candidates who visited during above period (n = 79), patients were excluded for a history of recent contact (within 3 months) with active TB patients, incomplete TB treatment, no consent, or no measured induration (n = 15). The control group consisted of 79 apparently healthy volunteers selected from a group of medical students and family members of admitted patients who had no previous history of TB and no known direct contact with TB patients. Hospital staff who had direct contact with TB patients, current hospital patients, chronic diseases or immunosuppressive drug treatments, or skin allergy disease or hypersensitivity were excluded from the control group.
All patients and the healthy volunteers were interviewed regarding their personal TB history. We performed a retrospective chart review of the medical history of each patient (e.g., the use of glucocorticoids and methotrexate [MTX], the presence of rheumatoid factor [RF], Disease Activity Score in 28 joints [DAS28], and disease duration). BCG vaccinations were verified by BCG scar identification and recorded. Chest radiographs were performed within 3 months before the QFT-Gold test and TST and were evaluated by an experienced radiologist.
All enrolled persons provided informed consent under Institutional Review Board-approved protocols at both Inje University Ilsan Paik Hospital and Ajou University Hospital.
Trained technicians performed the TST on the volar side of the forearm using the Mantoux method. A 2-TU dose of tuberculin-purified protein derivative (PPD RT23, Staten Serum Institute, Copenhagen, Denmark) was injected intradermally and read after 48 to 72 hours. The TST results were interpreted according to the current guidelines for LTBI in anti-TNF-╬▒ agent users in Korea. The QFT-Gold and TST were performed on the same day in each patient during the study period.
The QFT-Gold test was performed at the central laboratory of the Korea Green Cross Corporation according to the manufacturer's recommendations. Brief ly, whole blood specimens were incubated for 20 hours (overnight) at 37Ōäā in a humidified atmosphere. The IFN-╬│ level of a nil well was considered background and was subtracted from the results of the mitogen well and the antigen-stimulated wells. The test result was considered positive if the concentration of IFN-╬│ in the sample well after stimulation with early secretory antigen target 6 and/or culture filtrate protein 10 was Ōēź 0.35 IU/mL (after subtracting the nil well value), regardless of the result of the positive control (mitogen well). The test result was considered negative if the response to the specific antigens (after subtraction of the nil well value) was < 0.35 IU/mL and if the IFN-╬│ level of the positive control (after subtraction of the nil well value) was Ōēź 0.5 IU/mL. The test result was considered indeterminate if both antigen-stimulated sample wells were negative (< 0.35 IU/mL after subtraction of the nil well value) and if the value of the positive control well was < 0.5 IU/mL (after subtraction of the nil well value).
Demographic characteristics are presented as means ┬▒ SD (range) and categorical variables are expressed as frequencies. The cutoff value for the TST was based on an induration of 10 mm in diameter. The agreement between the QFT-Gold test and the TST was calculated using standard kappa statistics. The agreement between two tests in patients with RA taking glucocorticoids and MTX was also calculated. The test results were evaluated using Cohen's kappa (╬║), > 0.75 indicated good agreement, ╬║ = 0.4 to 0.75 indicated fair to good agreement, and ╬║ < 0.40 indicated poor agreement.
We subanalyzed the indeterminate QFT-Gold rates according to glucocorticoid and MTX dose, serum RF positivity rate, and history of BCG vaccination. All analyses were performed using the SPSS software version 18.0 (IBM Co., Armonk, NY, USA).
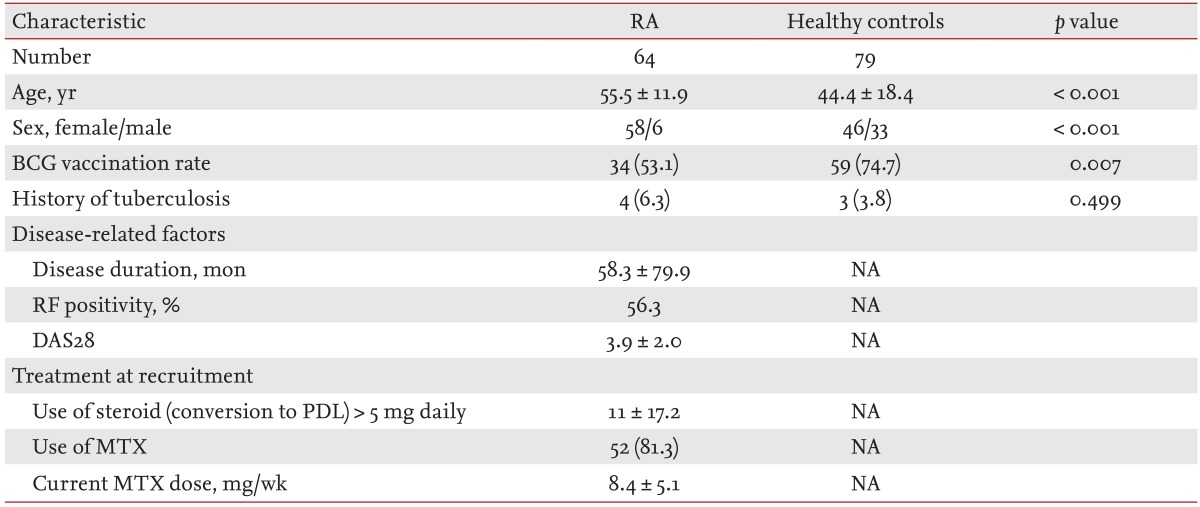
Demographic data and clinical characteristics of patients with RA and the control group are presented in Table 1. We enrolled 64 patients with RA (58 females, six males) and 79 healthy controls (46 females, 33 males). Compared with the RA patients, the mean age of the controls was younger (55.5 years vs. 44.4 years; p < 0.001), and the ratio of female patients was lower (90.6% vs. 58.2%; p < 0.001). Among the study populations, 34 patients (53.1%) with RA and 59 healthy controls (74.7%) had received the BCG vaccination (p = 0.007).
In the RA patients, the mean disease duration ┬▒ SD was 58.3 ┬▒ 79.9 months and the RF positivity rate was 56.3%. The mean DAS28-joint count ┬▒ SD was 3.9 ┬▒ 2.0. At the time of recruitment, the number (%) of RA patients taking glucocorticoids (conversion to prednisolone) in doses > 5 mg/day was 11 (17.2), and the daily mean doses of glucocorticoids were 3.2 ┬▒ 3.8 mg. The number (%) of patients taking MTX was 52 (81.3), and their current MTX doses were 8.4 ┬▒ 5.1 mg/week.
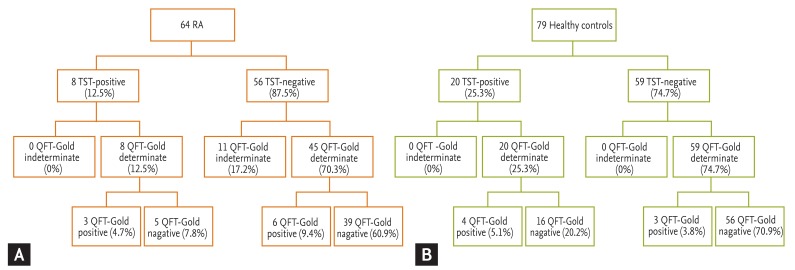
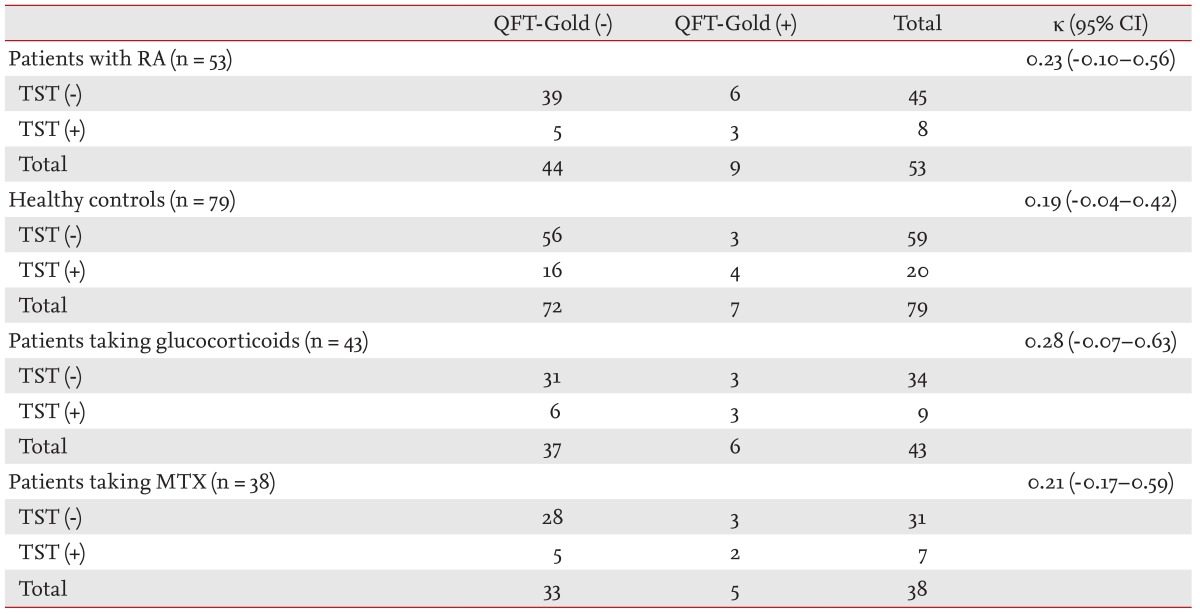
In the evaluation of both RA patients and healthy control for whom both test results were available, indeterminate QFT-Gold results were observed in 11/143 individuals (7.7%). Interestingly, all indeterminate QFT-Gold results were in patients with RA who had negative TST results (Fig. 1). We did not observe indeterminate QFT-Gold results in the healthy controls or in patients with RA who had positive TST results. In the healthy controls, the TST positivity rate (25.3%) was higher than that of the QFT-Gold test (8.9%), whereas in RA patients, the TST positivity rate (12.5%) was similar to the QFT-Gold positivity rate (14.1%) (Table 2, Fig. 1).
We excluded the results of 11 RA patients whose QFT-Gold test yielded indeterminate results in our agreement analyses. The overall agreement between the QFT-Gold and TST results was low, showing positive or negative concordance in 102/132 individuals (77.3%). Using a induration diameter of 10 mm as the TST cutoff value, the QFT-Gold and the TST results demonstrated 79.2% agreement (╬║ = 0.23) in the RA patients and 75.9% agreement (╬║ = 0.19) in the healthy controls. In RA patients taking glucocorticoids and MTX, the agreement rates between the QFT-Gold and the TST tests were 79.1% (╬║ = 0.28) and 78.9% (╬║ = 0.21), respectively (Table 2).
Using an induration diameter of 5 mm as the TST cutoff value, the agreement between the two test results was 28/53 (52.8%) and ╬║ = 0.05 in patients with RA and 45/79 (60.0%) and ╬║ = 0.05 in healthy controls (Table 3). Using an induration diameter of 15 mm as the TST cutoff value, the agreement between the two tests was 44/53 (83.0%) and ╬║ = 0.23 in patients with RA and 67/79 (84.8%) and ╬║ = 0.06 in the healthy controls (Table 3).
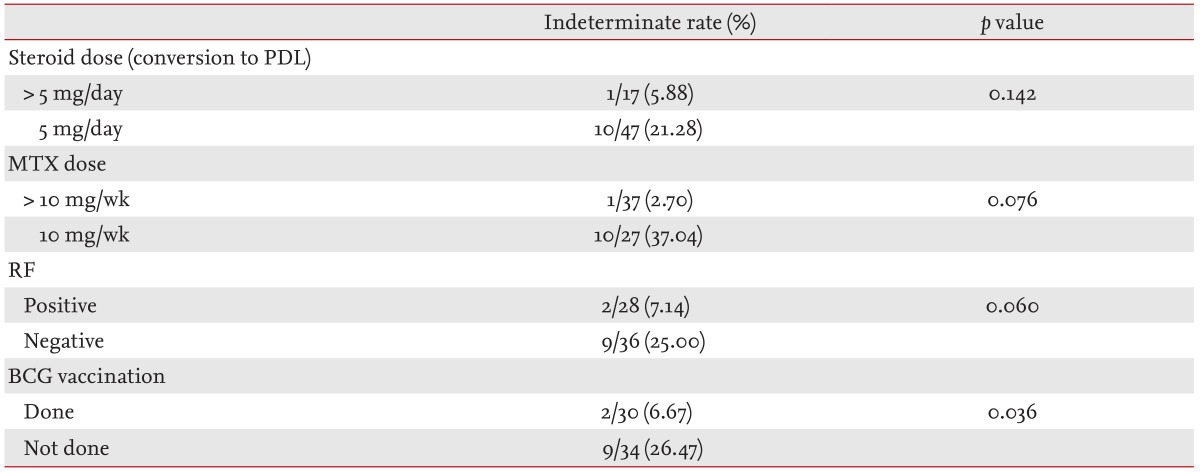
Of the 64 patients with RA, including those with indeterminate QFT-Gold results, we sub-analyzed the indeterminate QFT-Gold rates according to RF, MTX and glucocorticoid medication, and history of BCG vaccination (Table 4). In patients who had not received BCG vaccination, the rate of indeterminate QFT-Gold findings was significantly higher. No correlation was found between current glucocorticoid and MTX doses and RF positivity.
In this study, we found poor agreement between the results of the QFT-Gold test and the TST in patients with RA and healthy control groups (╬║ = 0.23 in patients with RA, ╬║ = 0.19 in healthy controls). Some studies have reported that there was a discrepancy between the two tests in South Korea, an intermediate TB burden country [11,12]. However, the reported agreement between IFN-╬│ assays and the TST was good (94% concordance in Denmark [╬║ = 0.87] and 89% in the United Kingdom [╬║ = 0.72]) in industrialized countries with low endemic TB [5,6]. Consequently, the incidence of TB is an important factor in agreement between the two tests.
The concordance between the QFT-Gold test and the TST was also affected by immune status. There was also poor concordance between the IFN-╬│ assays and the TST in LTBI diagnosis among individuals infected with the human immunodeficiency virus [14]. Agreement between the two tests in immunosuppressed patients with autoimmune diseases was also low [15]. In the case of patients with RA, some studies have shown generally poor concordance between IFN-╬│ assays and the TST regardless of TB burden [16,17]. In Korean patients with RA, similar studies showed poor agreement between the results of the two tests in immunocompromised patients [12,13].
The PPD antigen used in the TST resulted in false-positive results in individuals vaccinated with BCG. In Korea, the BCG vaccination is performed at birth and again at age 12 or 13 if the child proves to be a TST nonresponder. In our study, poor agreement between the two tests was investigated in the healthy control group. Not all participants in the healthy control group had a past history of BCG vaccination. Thus, considering the ages of the participants, the poor agreement between the two tests was likely caused by confounding effects of previous BCG vaccination [18-21].
We subanalyzed the agreement between the TST and QFT-Gold results according to DAS28 disease activity, C-reactive protein, glucocorticoids, and disease-modifying antirheumatic drugs (DMARDs). Agreement in the results of the two tests in patients with RA who were taking MTX or lef lunomide was poor (data not shown). Use of immunosuppressive agents and immune dysfunction may be related to RA with negative TST results [16,17].
The Korean guideline for the TST cutoff value for LTBI diagnosis before TNF-antagonist treatment is an induration > 10 mm [22]. By kappa analysis, we showed that the proper TST cutoff value for the highest concordance between QFT-Gold and TST results was 10 mm (Table 3). If we cannot rely on IFN-╬│ assays to diagnose LTBI, it is appropriate that we replace IFN-╬│ assay results with a TST cutoff value of 10 mm. Similar to the TST, the overall sensitivity of the QFT-Gold test was low, but its specificity was generally higher than that of the TST in this study.
In Korea, IFN-╬│ assays are generally considered alternatives or complementary to the TST in the diagnosis of LTBI. However, can IFN-╬│ assays completely replace the TST? One weak point of the QFT-Gold test is its high incidence of indeterminate results, which may indicate, and also arouse clinical suspicion of an immune defect. Some factors, such as very young or very old age, immune deficiencies, and use of oral steroids, may be associated with a higher rate of indeterminate results [23]. One study demonstrated an indeterminate result rate of 11% (43/383 cases), which was associated with immunosuppressive treatment [24]. Ferrara et al. [25] reported that the rate of indeterminate results for the QFT-Gold was 5% to 40%, and was significantly associated with elderly and immunocompromised patients. However, the high rate of indeterminate results of the QFT-Gold was anticipated to decrease with QFT-GIT [25]. The overall QFT-Gold indeterminate result rate was 7.7% (11/143 cases) in the current study. All indeterminate QFT-Gold results were from RA patients who were TST-negative and none was from the healthy controls. It seems reasonable to assume that an immunosuppressed condition would induce a depressed response to a T cell reaction. These results support the idea that the TST is often unreliable among immunocompromised patients [4] and that an indeterminate QFT-Gold result did not usually correlate with a positive TST result. Our results indicated that RA patients who had not received the BCG vaccination yielded higher indeterminate QFT-Gold rates compared with RA patients who had (Table 4). However, it is difficult to explain why BCG vaccination status should be related to indeterminate QFT-Gold results. Dose of glucocorticoids and MTX, positive rate of RF, and DAS28 did not inf luence the indeterminate QFT-Gold results in our study. These findings differed greatly from our expectations. Moreover, some investigators have suggested that a significant proportion of negative TST results could also show indeterminate QFT-Gold results. Considering the small sample size of our study, it is difficult to assess whether factors associated with immunity, such as the use of steroids and DMARDs, can induce indeterminate QFT-Gold results.
Another factor could be age (mean age 44.4 ┬▒ 18.4 vs. median age 28 [range, 25 to 36]). Because BCG vaccination was administered after infancy, TST administration > 15 years after vaccination did not result in significant reactions [19]. It is possible that the false-positive TST rates of our healthy controls were decreased because these controls had lower BCG vaccination rates and were older and less affected by the BCG vaccination than were the population in the study of Kang et al. [11]. To our knowledge, this is the first case-control study reported in Korea comparing two tests for the detection of LTBI in RA patients. We also investigated the clinical significance of indeterminate QFT-Gold results, which were commonly excluded in previous studies. Thus, investigations of clinical diagnoses (clinical symptoms, chest radiography, and/or chest computed tomography), patient medical histories (contact with TB patients), and environment (TB-endemic countries) are needed in the future.
Our study had some limitations. First, the sample sizes of both groups were small, which may have affected the ability to detect differences. Second, this study was performed in a country with an intermediate prevalence of TB. Thus, our results might be not generalizable to countries with lower or higher TB burdens. Third, there was a possibility of interobserver bias, because the TST induration measurements were performed by different observers in the two hospitals. Thus, further studies with larger sample sizes are required to confirm our findings.
We recommend that the relevance of individual risk factors for TB and the interpretation of TST and QFT-Gold test results should be considered in the diagnosis of LTBI. Clinicians should pay particular attention to immunosuppressed patients with negative TST and indeterminate QFT-Gold results. Furthermore, more weight should be given to individual clinical factors than to equivocal test results.
1. Poor agreement between the QuantiFERON-TB Gold (QFT-Gold) test and tuberculin skin test (TST) in patients with rheumatoid arthritis and healthy controls was found in this study.
2. We should pay particular attention to immunosuppressed patients with negative TST and indeterminate QFT-Gold results and always consider individual clinical factors more heavily than equivocal test results.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
1. Bieber J, Kavanaugh A. Consideration of the risk and treatment of tuberculosis in patients who have rheumatoid arthritis and receive biologic treatments. Rheum Dis Clin North Am 2004;30:257ŌĆō270PMID : 15172039.


2. World Health Organization. Tuberculosis (TB) [Internet]. Geneva (SW): World Health Organization, c2013;cited 2005 May 4. Available from: http://www.who.int/tb/en/.
3. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004;350:2060ŌĆō2067PMID : 15141044.


4. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis 1993;17:968ŌĆō975PMID : 8110954.


5. Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 2003;361:1168ŌĆō1173PMID : 12686038.


6. Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004;170:65ŌĆō69PMID : 15087297.


7. Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-gammabased assay using new antigens. Am J Respir Crit Care Med 2004;170:59ŌĆō64PMID : 15059788.


8. Mazurek GH, Villarino ME. CDC. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection: Centers for Disease Control and Prevention. MMWR Recomm Rep 2003;52(RR-2):15ŌĆō18PMID : 12583541.
9. Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005;54(RR-15):49ŌĆō55PMID : 16357824.

10. Menzies D. What does tuberculin reactivity after bacille Calmette-Guerin vaccination tell us? Clin Infect Dis 2000;31(Suppl 3):S71ŌĆōS74PMID : 11010826.


11. Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA 2005;293:2756ŌĆō2761PMID : 15941805.


12. Hur JA, Seo SH, Park SH, Cho CS, Kim HY. Discrepancy between the tuberculin skin test and the T-SPOT: TB for detecting latent mycobacterium tuberculosis infection in patients with rheumatoid arthritis. J Korean Rheum Assoc 2006;13:285ŌĆō290.
13. Park JH, Seo GY, Lee JS, Kim TH, Yoo DH. Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol 2009;36:2158ŌĆō2163PMID : 19723901.


14. Bartalesi F, Vicidomini S, Goletti D, et al. QuantiFERON-TB Gold and the TST are both useful for latent tuberculosis infection screening in autoimmune diseases. Eur Respir J 2009;33:586ŌĆō593PMID : 19047313.


15. Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis 2008;67:84ŌĆō90PMID : 17644549.


16. Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, et al. Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol 2008;35:776ŌĆō781PMID : 18398944.

17. Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum 2008;59:800ŌĆō806PMID : 18512714.


18. Huang YW, Shen GH, Lee JJ, Yang WT. Latent tuberculosis infection among close contacts of multidrug-resistant tuberculosis patients in central Taiwan. Int J Tuberc Lung Dis 2010;14:1430ŌĆō1435PMID : 20937183.

19. Shalabi NM, Houssen ME. Discrepancy between the tuberculin skin test and the levels of serum interferon-gamma in the diagnosis of tubercular infection in contacts. Clin Biochem 2009;42:1596ŌĆō1601PMID : 19732759.


20. Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 2002;57:804ŌĆō809PMID : 12200526.



21. Jeong SJ, Han SH, Kim CO, et al. Predictive factors for indeterminate result on the QuantiFERON test in an intermediate tuberculosis-burden country. J Infect 2011;62:347ŌĆō354PMID : 21414356.


22. Korea Food and Drug Administration. Guideline for diagnosis and treatment of latent tuberculosis infection in patient in patients treated with TNF blockers [Internet]. Cheongwon: Korea Food and Drug Administration, 2004;2013 Feb 1. Available from: http://www.mfds.go.kr/index.do?seq=4281&mid=70&cmd=v.
23. Ferrara G, Losi M, D'Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 2006;367:1328ŌĆō1334PMID : 16631911.


Figure┬Ā1
Flow-chart showing the distribution of QuantiFERON-TB (QFT-Gold) results in tuberculin skin test (TST)-positive and TST-negative patients with (A) rheumatoid arthritis (RA) and (B) healthy controls.
Table┬Ā2
Agreement between QuantiFERON-TB Gold and tuberculin skin rest results in enrolled participant and immunosupressive therapy




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



