 |
 |
| Korean J Intern Med > Volume 32(2); 2017 > Article |
|
See editorial "Serum vascular endothelial growth factor as a marker of asthma exacerbation" on page 258.
Abstract
Background/Aims
Vascular endothelial growth factor (VEGF) is an important mediator of angiogenesis. However, little is known about the potential use of serum levels of VEGF as a biomarker for asthma. We investigated the differences in VEGF levels among normal controls, stable asthma patients, and those with exacerbation of acute asthma. All subjects were young males.
Methods
We measured VEGF levels in each patient group, and examined any serial changes in those with acute exacerbation.
Results
VEGF levels were significantly higher in stable asthmatic patients and even more so in acute asthmatic patients, compared to healthy controls. However, there was no correlation between VEGF levels and forced expiratory volume in 1 second in patients with stable asthma. In addition, there were no correlations between VEGF levels and asthma control test scores. In patients with acute exacerbation, VEGF levels significantly increased during the acute period; their levels decreased gradually at 7 and 14 days after treatment.
Conclusions
Compared to normal control patients, the serum levels of VEGF were elevated in stable asthma patients and even more elevated in patients with acute exacerbation. However, the role of VEGF as a biomarker in stable asthma is limited. In patients with acute exacerbation, VEGF levels were associated with clinical improvements.
Asthma is a chronic inflammatory disease of the airways characterized by structural changes including subepithelial fibrosis, smooth muscle cell hypertrophy, epithelial cell metaplasia, and angiogenesis [1]. Remodeling of microvasculature (e.g., proliferation of new vessels) and increased vascular areas of the medium and small airways lead to increased blood flow and microvascular permeability. This contributes to thickened, engorged, and edematous airway walls, which result in the narrowing of the airway lumen [2-4]; this, in turn, results in increased delivery of inflammatory mediators to the airways, leading to increased bronchial hyper-reactivity and airflow obstruction [5].
Increased airway vascularity is seen even in mild asthma [2] and is associated with a greater expression of vascular endothelial growth factor (VEGF) [6]. VEGF is the most potent angiogenic mediator. It is a key regulator of blood vessel growth in the airways of asthma patients, which it does by promoting proliferation and differentiation of endothelial cells, inducing vascular leakage and increased permeability [7].
Various studies have proved that VEGF is involved in the pathogenesis of asthma [8-11]. However, its potential role as a biomarker is still limited. Moreover, there have only been a few studies on the levels of VEGF in the serum of asthma patients. Hence, we investigated the clinical significance of serum levels of VEGF in asthmatic patients.
This study was a prospective observational study. Patient data were collected with this in mind. The study was undertaken at the Armed Forces Yangju Hospital and Armed Forces Capital Hospital, South Korea, between June 2008 and January 2011. The criteria for inclusion were (1) patients over 18 years of age, (2) diagnosis of asthma by a pulmonary or allergy specialist, and (3) written informed consent. The study was approved by the Institutional Review Board of the Armed Forces Yangju Hospital and Armed Forces Capital Hospital. Fully informed, written consent was obtained from each subject.
A total of 124 young, male patients with asthma were recruited. Asthma was diagnosed by a pulmonary or allergy specialist from our the Armed Forces Yangju Hospital and Armed Forces Capital Hospital according to the criteria of the Global Initiative for Asthma [12].
Among these patients, 104 had visited the outpatient clinic with stable asthma. Twenty patients had acute exacerbation and were admitted to the hospital due to exacerbation of their asthma. All patients with acute exacerbation received the standard therapy with oxygen and inhaled bronchodilators, including a short-acting beta agonist, via a nebulizer. A systemic corticosteroid (1 mg prednisolone/kg or equivalent) was administered and tapered during admission.
In addition, 58 normal healthy subjects were recruited for this study; these were people who had visited the hospital for a routine check-up and who had no underlying disease. Baseline clinical data including age, height, and weight were collected. VEGF levels in the serum and total immunoglobulin E (IgE) levels in the serum were measured on the day of the hospital visit. For the stable asthma patients, the asthma control test (ACT) score was also measured.
Pulmonary function tests (PFTs) were performed following American Thoracic Society/European Respiratory Society guidelines in a laboratory licensed for testing acute asthma patients, by technicians experienced in lung function testing. Spirometry was performed according to current recommendations [13,14]. For stable asthma, PFT was performed on the same day the serum sample was collected. For patients with acute exacerbation, the forced expiratory volume in 1 second (FEV1) was measured on day 1 of exacerbation, and at days 3, 7, and 14.
In the stable asthma patients, serum samples were obtained on the day the patient visited the outpatient clinic. In the acute exacerbation patients, initial serum samples were obtained on day 1 of exacerbation, and then on days 3, 7, and 14 during their admission.
The levels of VEGF were determined by enzyme immunoassays according to the manufacturerās protocol (R&D Systems Inc., Minneapolis, MN, USA). Sensitivities for VEGF assays were 9 pg/mL.
Because the majority of the data did not follow a normal distribution, a nonparametric statistical method was used for the analysis. Continuous variables were analyzed using Mann-Whitney U tests. Spearman correlation coefficient, rho (Ļ), was used to assess whether there was a relationship between VEGF and other parameters. Comparisons of the three groups of patients were analyzed using a Kruskal-Wallis test. A Wilcoxon signed-rank test was used for further analysis in patients with acute asthma exacerbation (on days 1, 3, 7, and 14). All tests were two-sided and a p < 0.05 was considered to be statistically significant. All analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
The clinical characteristics of each group are shown in Table 1. There were no significant differences in age, height, weight, and body mass index among the groups. Significant differences in VEGF (p < 0.01) and IgE (p < 0.01) levels were found among all three groups.
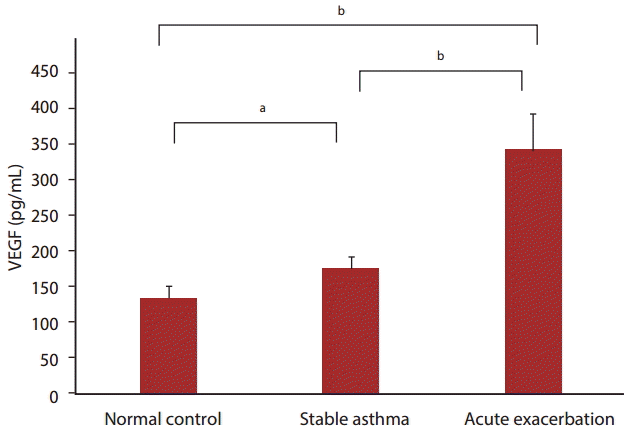
The level of VEGF in serum was higher in stable asthmatic patients (177.1 Ā± 13.5 pg/mL) and even much higher in acute exacerbation patients (339.2 Ā± 50.4 pg/mL, at day 3 of exacerbation) compared to healthy controls (134.5 Ā± 16.8 pg/mL). The serum level of VEGF in stable asthma was significantly higher than in normal control (p < 0.05). The level of VEGF in acute exacerbation was significantly higher than in stable asthma (p < 0.01) and normal control (p < 0.01) (Fig. 1).
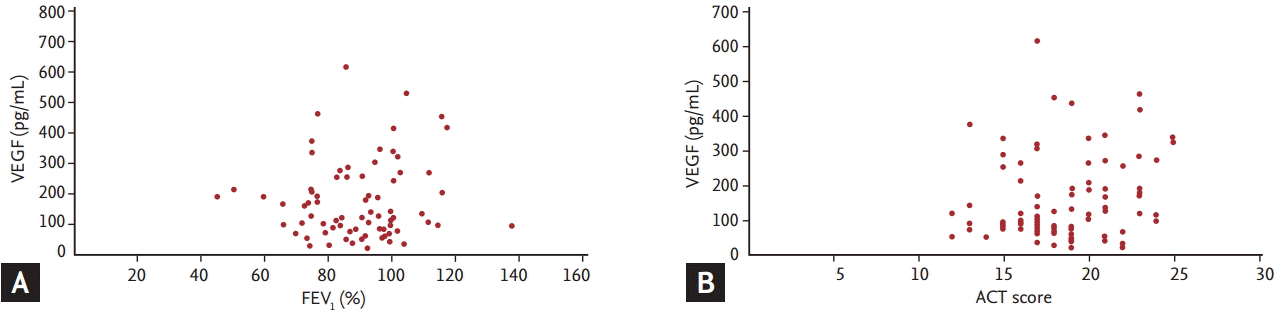
Correlations between VEGF and FEV1 or ACT score were analyzed in the stable asthma patients. No significant correlations were found between VEGF and FEV1 (Ļ = 0.06, p = 0.60) or between VEGF and the ACT score (Ļ = 0.20, p = 0.06) (Fig. 2).
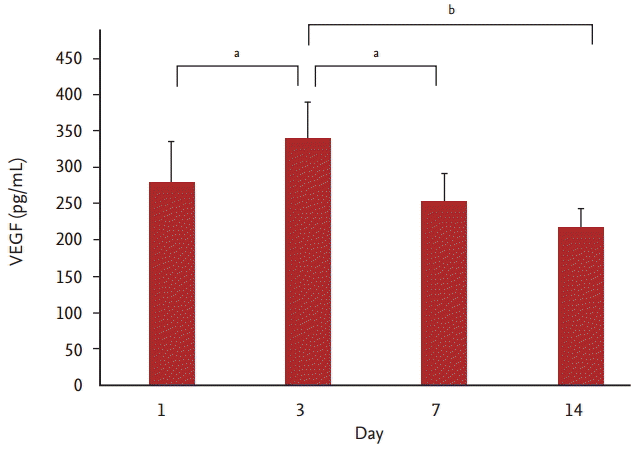
VEGF levels in the acute exacerbation group before and during treatment are shown in Fig. 3. The mean level on day 1 was 278.6 Ā± 56.5 pg/mL; this increased to the maximum level on day 3 (339.2 Ā± 50.4 pg/mL) and then decreased by day 7 (253.9 Ā± 37.2 pg/mL) and was even lower by day 14 (218 Ā± 25.7 pg/mL). The mean level on day 3 was significantly higher than on day 1 (p < 0.05). Compared to day 3, the levels on days 7 and 14 were significantly lower (p < 0.05 and p < 0.01, respectively) (Fig. 3).
The FEV1 on day 1 was 67.1% Ā± 5.9%; this increased gradually on day 3 (76.1% Ā± 4.1%) and even further by days 7 (88.8% Ā± 5.1%) and 14 (91.8% Ā± 3.6%). Compared with day 1, FEV1 (%) in day 7 and 14 was significantly higher (p < 0.05, p < 0.01, respectively). Compared with day 3, FEV1 (%) in day 7 and 14 was also significantly higher (p < 0.05, p < 0.01, respectively).
VEGF plays a fundamental role in angiogenesis of the airways and is synthesized by the alveolar epithelial cells, bronchial epithelial cells, smooth muscle cells, fibroblasts, and alveolar macrophages [8,9]. Furthermore, VEGF promotes allergic inflammation and plays an important role in Th2 inflammation. It induces eosinophilic inflammation, mucus metaplasia, subepithelial fibrosis, myocyte hyperplasia, dendritic cell activation, and airway hyper-responsiveness via interleukin (IL)-13-dependent and IL-13-independent mechanisms [10]. In addition, Th2 cytokines such as IL-4, IL-5, and IL-13 induce structural cells to produce VEGF, which in turn enhances allergen-induced inflammation and consequent remodeling [11].
Several studies have demonstrated the involvement of VEGF in asthmatic subjects. For example, levels of VEGF are higher in induced sputum [15-19] and bronchoalveolar lavage (BAL) fluid [20] from asthma patients, in whom they are correlated with disease severity. Hoshino et al. [6] reported more VEGF-positive cells in bronchial biopsy samples from asthmatic airways and concluded that this was associated with the degree of airway vascularity.
However, measuring VEGF in induced sputum or BAL fluid is not easy in clinical practice. It is much easier and more convenient to measure them from blood samples. Thus, the level of VEGF in blood sample is more adequate as biomarker in real practice. However, there have only been a few studies which measured VEGF in blood of asthma patients. To the best of our knowledge, only three studies have attempted this on adult asthma patients [21-23]. Moreover, among three studies, only one study [23] showed the relationship between VEGF and FEV1. Our study is unique in that we have shown the limited role of serum VEGF in stable asthma patients. Compared with previous similar study [23], this study has merit in that the number of stable patients is much bigger (8 vs. 104). Moreover, this is first study to show that there is no significant correlation between serum VEGF and ACT score. ACT score is a key biomarker when deciding to step up or down. The role of ACT is as important as lung function in the management of asthma patients. Thus, to be a meaningful biomarker, VEGF levels in the serum should correlate with ACT. Unfortunately, in our study, this result was found to be negative.
In previous studies [6,17], VEGF levels in sputum or bronchial biopsies was were correlated with disease severity. However, in our study, VEGF in the blood was not correlated with disease severity. The reason for this discrepancy is not yet known. One possible explanation could be differences in VEGF level between lung tissue and blood. For example, in Zou et al. [21], the levels of VEGF in the sputum were higher in patients with moderate exacerbation than in those with mild exacerbation, whereas the levels in serum were higher in those with mild exacerbation. This may suggest that the level of VEGF in sputum may not be exactly correlated with serum. Similarly, in the study of Bikov et al. [22], the level of free plasma VEGF differed according to the degree of pregnancy. However, the level of VEGF in exhaled breath condensate did not. This also suggests that the level of systemic VEGF might be different from lung VEGF. Actually, the only study which showed correlation between blood VEGF level and severity (FEV1) in adult asthma is Lee et al.ās one [23]. However, in fact, they did not compare two parameters in stable asthma patients. Instead, they included all enrolled subjects (normal controls, stable asthma patients, and exacerbation patients) in the analyses. Thus, their results do not indicate the potential role of VEGF as a biomarker in stable asthma.
We found that VEGF levels in serum were significantly increased in stable asthmatic patients, and even more so in acute asthmatic patients, in agreement with that previous study [23]. From these results we can conclude that VEGF is involved in the pathogenesis of asthma and may play a role in the exacerbation of acute asthma. In the exacerbation group, VEGF levels significantly increased during the acute period. After therapy including methylprednisolone, the levels significantly decreased during the 2-week remission period. Therefore, in this patient group, VEGF levels in the serum can be considered a marker of clinical improvement.
Our study is important in that it used a much larger sample size compared to the previous similar study [23]. Furthermore, our subjects were a homogenous group of young males, and therefore confounding factors such as COPD could be ruled out and the subjects could be considered pure asthma patients.
However, there are several limitations to our study. First, as the study was conducted at army-based hospitals, the study samples had a gender bias (males only). Further clinical studies that include various ages and sexes are needed. Second, we did not adjust for other possible factors that can affect VEGF levels. As we measured systemic VEGF, not levels in lung, this level can be affected by other systemic factors. For example, VEGF can be increased in rhinovirus infection [24], smoking [25], and Barrettās esophagus [26]. It can be decreased in glucocorticoid treatment [24] and emphysema [27]. Such factors could have affected our results. Third, VEGF is elevated in exercise-induced asthma (EIA) and plays an important role in the pathogenesis of EIA [15,28,29]. However, we did not perform an exercise challenge test and thus could not provide information regarding EIA.
In conclusion, VEGF levels in the serum are elevated in stable asthma patients, and even more elevated in patients with acute exacerbation of asthma. However, the role of VEGF as a biomarker in stable asthma is limited. In patients with acute exacerbation, VEGF levels correlate with clinical improvements.
1. Serum vascular endothelial growth factor (VEGF) is elevated in stable asthma patients and more elevated in patients with acute exacerbation compared to normal control.
2. The role of VEGF as a biomarker in stable asthma is limited.
3. In patients with acute exacerbation of asthma, VEGF levels correlate with clinical improvements.
FigureĀ 1.
Comparison of serum vascular endothelial growth factor (VEGF) levels in control, stable asthma, and acute asthma exacerbation subjects. Values are presented as mean Ā± SEM. ap < 0.05, bp < 0.01.
FigureĀ 2.
Correlation between serum vascular endothelial growth factor (VEGF) and (A) the forced expiratory volume in 1 second (FEV1) or (B) asthma control test (ACT) score in stable asthma subjects.
FigureĀ 3.
Time course of serum vascular endothelial growth factor (VEGF) in acute asthma exacerbation subjects. Values are presented as mean Ā± SEM. ap < 0.05, bp < 0.01.
TableĀ 1.
Baseline characteristics
| Characteristic | Control (n = 58) | Stable asthma (n = 104) | Acute exacerbation (n = 20) | p value |
|---|---|---|---|---|
| Age, yr | 20.5 Ā± 0.2 | 20.4 Ā± 0.1 | 20.7 Ā± 0.4 | 0.56 |
| Height, cm | 175.1 Ā± 0.8 | 174.6 Ā± 0.6 | 172.9 Ā± 1.9 | 0.38 |
| Weight, kg | 68.7 Ā± 1.3 | 71.8 Ā± 1.2 | 70.7 Ā± 3.0 | 0.51 |
| Body mass index, kg/m2 | 22.4 Ā± 0.3 | 23.5 Ā± 0.4 | 23.6 Ā± 0.9 | 0.23 |
| VEGF, pg/mL | 134.5 Ā± 16.8 | 177.1 Ā± 13.5 | 339.2 Ā± 50.4a | < 0.01 |
| Total IgE, U/mL | 157.5 Ā± 25.2 | 389.7 Ā± 51.3 | 335.6 Ā± 47.7 | < 0.01 |
| ACT score | - | 18.6 Ā± 0.3 | - |
REFERENCES
1. Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 2010;36:1174ā1184.


2. Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229ā233.


4. Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res 2010;11:132.



5. Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 2010;17:e85āe93.




6. Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001;107:295ā301.


7. Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 2010;65:946ā958.


8. Ribatti D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol 2005;128:303ā309.


9. Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol (1985) 2004;97:1605ā1617.


10. Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095ā1103.



11. Wen FQ, Liu X, Manda W, et al. TH2 cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol 2003;111:1307ā1318.


12. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143ā178.


13. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319ā338.


14. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511ā522.


15. Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax 2002;57:885ā888.



16. Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol 2002;110:571ā575.


17. Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy 2003;33:595ā599.


18. Papadaki G, Bakakos P, Kostikas K, et al. Vascular endothelial growth factor and cysteinyl leukotrienes in sputum supernatant of patients with asthma. Respir Med 2013;107:1339ā1345.


19. Abdel-Rahman AM, el-Sahrigy SA, Bakr SI. A comparative study of two angiogenic factors: vascular endothelial growth factor and angiogenin in induced sputum from asthmatic children in acute attack. Chest 2006;129:266ā271.


20. Feltis BN, Wignarajah D, Zheng L, et al. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med 2006;173:1201ā1207.


21. Zou H, Fang QH, Ma YM, Wang XY. Analysis of growth factors in serum and induced sputum from patients with asthma. Exp Ther Med 2014;8:573ā578.



22. Bikov A, Bohacs A, Eszes N, et al. Circulating and exhaled vascular endothelial growth factor in asthmatic pregnancy. Biomarkers 2012;17:648ā654.


23. Lee KY, Lee KS, Park SJ, et al. Clinical significance of plasma and serum vascular endothelial growth factor in asthma. J Asthma 2008;45:735ā739.


24. Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep 2013;13:1ā9.


25. Farid Hosseini R, Jabbari Azad F, Yousefzadeh H, et al. Serum levels of vascular endothelial growth factor in chronic obstructive pulmonary disease. Med J Islam Repub Iran 2014;28:85.


26. Zampeli E, Karamanolis G, Morfopoulos G, et al. Increased expression of VEGF, COX-2, and Ki-67 in Barrettās esophagus: does the length matter? Dig Dis Sci 2012;57:1190ā1196.


27. Lee CG, Ma B, Takyar S, et al. Studies of vascular endothelial growth factor in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2011;8:512ā515.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



