INTRODUCTION
Albumin is an effective plasma volume expander due to its high oncotic activity and prolonged half-life in the intravascular compartment. Considering these factors, it is not surprising that albumin has been used for many years in the management of patients with cirrhosis and ascites [1]. Evidence has been presented in support of albumin use in the management of complications of cirrhosis, but arguments against such use have also been put forth, especially because albumin infusions are costly and this treatment has not been demonstrated to improve survival [2]. The debate has been fostered by the results of a recent meta-analysis showing that albumin administration may increase mortality in critically ill patients [3].
The main physiologic function of albumin is to maintain colloid osmotic pressure, but in the past few years many other functions have been recognized. These include ligand binding and transport of various molecules, in addition to antioxidant and anti-inflammatory actions [4]. These functions of albumin could be applied to various clinical situations, including septic shock. Patients with cirrhosis, especially those in the advanced decompensated stage, exhibit effective arterial hypovolemia and are prone to the development of sepsis. Thus, the possible indications for albumin use in these cirrhotic patients are rapidly expanding. This review discusses the physiologic actions of albumin and the potential benefits and pitfalls of albumin use in patients with end-stage liver disease.
PHYSIOLOGIC FUNCTIONS OF ALBUMIN
Colloidal osmotic pressure
Albumin is predominantly an extravascular protein, and its serum concentration is -40 g/L, suggesting a total intravascular mass of -120 g [5]. The interstitial concentration is lower (14 g/L) and varies among anatomic regions. However, the total extravascular mass is -160 g [5]. Some is easily mobilized from loose interstitial tissues, whereas the remainder is tightly bound. Albumin appears to circulate from the intravascular to extravascular spaces, and the transcapillary escape rate is determined by the capillary and interstitial free albumin concentrations, microvascular permeability to albumin, movement of solvents and solutes, and transcapillary electrical charge. In patients with hypoalbuminemia (especially when associated with inflammation or sepsis), whose capillaries are known to be hyper-permeable, leakage of albumin into the interstitial space draws water and produces edema [6].
Transport
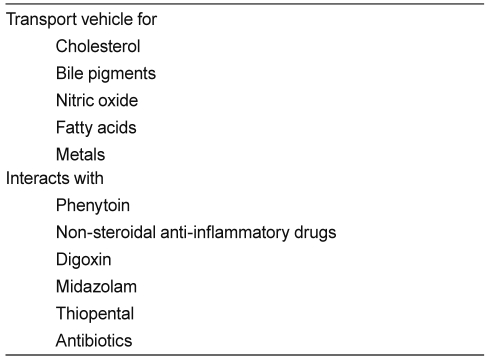
Albumin has a strong negative charge, but binds weakly and reversibly to both cations and anions. Therefore, it functions as a transport molecule for a large number of metabolites, including fatty acids, ions, thyroxine, bilirubin, and amino acids (Table 1). Albumin also binds covalently and irreversibly with d-glucose and d-galactose. The glycosylation of albumin, which is to a certain extent age-dependent, affects its charge and, therefore, may influence capillary permeability characteristics [5].
Antioxidant effects
Albumin is the major extracellular source of thiols. These sulphydryl groups are scavengers of reactive oxygen and nitrogen species. Albumin can also limit the production of reactive oxidative species by binding free copper, an ion known to be particularly important in accelerating the production of free radicals. In sepsis, the administration of human albumin led to significantly increased levels of total plasma thiol levels. Unlike albumin concentrations, however, which fell significantly between 5 minutes and 4 hours following administration, thiol remained significantly elevated for up to 18 hours following albumin administration [7]. These results suggest that the increase in plasma protein thiols associated with albumin administration is sustained long-term compared with plasma albumin levels, which is indicative of a beneficial albumin-mediated thiol exchange in the plasma of these septic patients. Also, albumin may in this way influence redox balance, which has several important implications for other indices of critical illness, including capillary permeability, cell signaling processes, and drug metabolism and transport [4].
Endothelial stabilization
Albumin's ability to reduce injury to the endothelium caused by reactive oxygen and nitrogen species means that it may stabilize the endothelium and help to maintain capillary permeability. Albumin also interferes with neutrophil adhesion to the capillary endothelium [8], thereby reducing inflammation and aiding the maintenance of endothelial integrity.
Pharmacologic interactions and drug binding
Drugs with which albumin interacts are clinically significant owing to their highly protein-bound state and low margins of safety, and include warfarin, phenytoin, non-steroidal anti-inflammatory drugs, digoxin, midazolam, thiopental, and several antibiotics. The distribution volume of drugs bound to albumin may increase in hypoalbuminemia, reducing their efficacy [5]. Administration of mixtures of loop diuretics with albumin has therefore been advocated, although this has been shown to be ineffective in cirrhotic patients with ascites.
PATHOGENESIS OF ASCITES AND RENAL DYSFUNCTION IN CIRRHOSIS
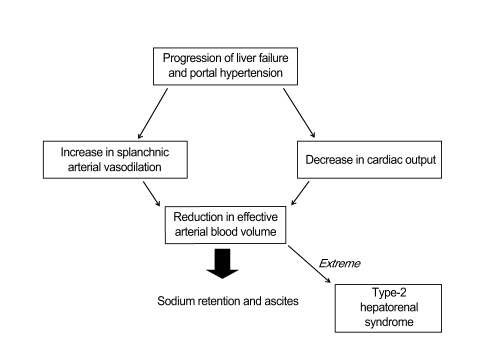
Evidence strongly indicates that renal dysfunction and ascites formation in cirrhosis are the final consequences of circulatory dysfunction. This is characterized by marked splanchnic arterial vasodilatation, causing a reduction in effective arterial blood volume and the homeostatic activation of vasoconstrictor and anti-natriuretic mechanisms. The exact mechanism(s) leading to this vasodilatation are incompletely understood, but may involve increased synthesis/activity of vasodilator factors, including nitric oxide and vasodilator peptides [9,10]. These splanchnic arterial vasodilatations are likely responsible not only for the reduction in total systemic vascular resistance, but also for an abnormal distribution of blood volume with reduction of effective arterial blood volume (Fig. 1). Reduction of effective arterial volume stimulates the renin-angiotensin system and induces vasopressin release, which leads to continuous renal sodium and water retention, and ascites formation [11]. In contrast, no evidence supports a role for reduced vascular oncotic pressure due to hypoalbuminemia in the pathogenesis of ascites. Renal dysfunction in cirrhosis is of great clinical importance because its intensity correlates with prognosis [1].
ALBUMIN USE IN GENERAL CLINICAL PRACTICE
Albumin has been used in many clinical scenarios, especially those requiring the improvement of colloid osmotic pressure (e.g., shock and sepsis) [12]. However, since the Cochrane Review reported that albumin administration to critically ill patients might increase the risk of death [3], the use of albumin in clinical practice, especially in the critical-care setting, has been controversial [13]. In addition, a large clinical trial that included 7,000 critically ill patients showed that normal saline was as effective as 4% albumin as a resuscitation fluid; no difference in morbidity, length of stay in the critical-care unit or hospital, or survival was found [14,15]. With the added concerns of potential transmission of known and unknown infections via administration of human albumin [16] and its high cost, the use of albumin in general clinical practice remains controversial [6].
ALBUMIN USE IN LIVER CIRRHOSIS
For the management of cirrhotic ascites
The standard treatment for cirrhotic ascites is sodium restriction and diuretic therapy. One randomized, controlled trial assessed the effects of albumin plus standard diuretic therapy in cirrhotic patients with ascites; weekly infusions of 25 g albumin produced a significantly better diuretic response, shorter hospital stays, and a lower likelihood of readmission to hospital than treatment with standard therapy [17]. Suppression of the activity of anti-natriuretic systems, particularly the renin-angiotensin-aldosterone system, probably accounts for an increase in the natriuretic response to diuretics with repeated albumin infusions [18]. Survival, however, was not affected by the addition of albumin [6]. Moreover, compared with the simple performance of paracentesis in a day-care unit, the logistic problems of intravenous albumin administration on a weekly basis, and its lack of cost-effectiveness, render this indication unjust and impractical in clinical practice [19]. Infusions of albumin plus diuretic therapy, therefore, cannot be recommended as the standard of care for these patients [6].
For the prevention of renal dysfunction in patients with cirrhosis and ascites
To date, two different situations that may further impair circulatory function in cirrhotic patients with ascites have been identified: large-volume paracentesis and spontaneous bacterial peritonitis (SBP).
Large-volume paracentesis
The removal of large amounts of ascitic fluid is characterized by early favorable hemodynamic effects, with suppression of vasoconstrictor and anti-natriuretic factors and increased plasma natriuretic peptide levels. However, this is followed by a second phase characterized by marked activation of vasoconstrictor and anti-natriuretic factors in the absence of changes in plasma volume, consistent with the impairment of effective arterial blood volume [20]. This paracentesis-induced circulatory dysfunction (PCD) occurs in most patients treated with large taps (> 5 L), is not spontaneously reversible, and is associated with the impairment of renal function and decreased survival [11,21,22].
The prevention of PCD is the most controversial indication for albumin use, but the most important quantitatively. In the single randomized, controlled trial that compared paracentesis plus albumin infusion (10 g/L of ascitic fluid removed) with paracentesis alone, the incidence of circulatory dysfunction was significantly decreased in the paracentesis plus albumin group (16%) compared with the paracentesis-only group (30%) [21]. Other plasma expanders (e.g., dextran 70) were compared with albumin, and albumin was more effective only when > 5 L paracentesis was performed [22]. A single relatively large-volume paracentesis (< 5 L) without albumin replacement was shown to have no deleterious consequence or adverse disturbance in systemic or renal hemodynamics [23]. As severely critically ill cirrhotic patients usually stay > 1 day in hospital, repeated small-volume paracentesis (< 5 L) will lessen the need for albumin infusion. In addition, no study to date has demonstrated a significant advantage of total paracentesis compared with repeated smaller-volume paracentesis [19].
The American Association for the Study of Liver Disease (AASLD) recommended that albumin infusion should be given at a dose of 6-8 g/L ascites fluid removed for paracentesis volumes > 5-6 L. Fifty percent should be given in the first hour (maximum 170 mL/hr) and the remainder in the next 6 hours [24]. The uses of albumin substitute fluids, such as hydroxyethyl starch (HES), which can prevent circulatory failure after paracentesis remains controversial [2].
Spontaneous bacterial peritonitis
Patients with SBP risk systemic hemodynamic parameter deterioration, with further arterial and splanchnic vasodilatation. These patients are thus at high risk of developing renal insufficiency [25]. In a study of the effect of albumin infusion on renal function and survival in patients with SBP, 126 patients were randomly allocated to receive either cefotaxime alone or cefotaxime plus albumin infusions [26]. Albumin was given at a dose of 1.5 g/kg body weight within 6 hours of SBP diagnosis, followed by a further infusion of 1 g/kg body weight on day 3. This strategy resulted in a large albumin infusion, for example, 105 g on day 1 and 70 g on day 3 in a 70-kg patient. Patients who were given cefotaxime plus albumin infusions showed no increase in plasma renin activity, a decreased incidence of renal failure, and a decreased mortality rate (from 29% to 10%) compared with patients who were given cefotaxime alone. Criticisms of this study were the inclusion of more sick control patients than those who received albumin with cefotaxime. Secondly, a central venous pressure line was inserted only in patients with signs of hypovolemia. Thirdly, no comparison was made with other, less expensive, plasma volume expanders. These results suggest that the benefits of albumin infusion apply only to a subset of patients with more advanced liver disease. The amount of albumin used in this study was also high, making this strategic therapy costly and impractical [19]. To address these criticisms, the authors of the original study compared infusions of albumin with infusions of HES for the prevention of renal failure in patients with SBP [27]. The findings supported the superiority of albumin in preventing the development of renal failure in patients with SBP. Another study comparing albumin, crystalloid fluid, and artificial colloid corroborated this finding [28]. Although albumin has a significant role in patients with SBP and severely disturbed liver and renal functions, its use continues to be debated because of the relatively high dose and cost [19,24]. Nevertheless, the development of renal failure in cirrhotic patients with SBP carries a high risk of morbidity and mortality, so the use of albumin infusion as an adjunctive therapy in the treatment of patients with SBP will continue until further trials are completed.
For the management of renal dysfunction in patients with cirrhosis and ascites
The administration of albumin to patients with cirrhosis and ascites causes an increase in total blood volume, followed by a moderate reduction, but not normalization, of the activity of vasoconstrictor and anti-natriuretic systems. These circulatory changes are associated with favorable effects on renal function. However, these renal effects are modest and limited only to patients with normal or slightly impaired renal function, whereas patients with severe renal dysfunction show no beneficial response [29-32]. Albumin infusion alone fails to consistently improve circulatory and renal functions because albumin cannot increase effective arterial blood volume efficiently due to the extreme splanchnic vasodilatation present in these cirrhotic patients [1].
Hepatorenal syndrome (HRS) is characterized by very low arterial pressure and total systemic vascular resistance, marked over-activity of vasoconstrictor factors, and marked arterial vasoconstriction in the kidney and other vascular territories (muscle, skin, and brain) [33]. For many years, HRS was considered a terminal irreversible event in patients with decompensated cirrhosis. However, it was demonstrated that patients with type 1 HRS may be effectively treated with a combination of vasoconstrictor and plasma volume expansion [34]. The use of albumin appears to increase the efficacy of vasoconstrictor drugs. Two studies have shown that the treatment of cirrhotic patients with HRS for several days or weeks with a combination of vasoconstrictors and plasma volume expansion with albumin results in a marked improvement in circulatory and renal functions in most cases, with normalization of plasma levels of vasoconstrictor factors and serum creatinine [35,36]. However, the need for a plasma expander agent as a co-therapy remains unclear. The administration of vasoconstrictors with albumin has been shown to reverse type 1 HRS and normalize renal function in 60-70% of treated patients. However, these studies included only small numbers of patients, some of whom were not randomized, and the impact on long-term (> 1 month) survival has not been shown. Available data on the treatment of type 2 HRS are much scarcer than for type 1 HRS [19]. Whether albumin is required to achieve the beneficial effect of vasoconstrictor therapy in HRS is not known. However, albumin likely improves the therapeutic efficacy of vasoconstrictors, as the improvement in circulatory and renal functions is more marked in patients treated with terlipressin and albumin than that in patients treated with terlipressin alone. Given that albumin has volume-expanding, ligand-binding, and antioxidant properties, it seems prudent to use albumin infusions in the treatment of HRS unless there is evidence that albumin actually does some harm. Currently, the AASLD recommends that albumin infusion plus administration of vasoactive drugs such as octreotide and midodrine should be considered in the treatment of type 1 HRS (level II-1).
Molecular adsorbent recirculating system
The recent development of the molecular absorbent recirculating system (MARS), an albumin dialysis that removes albumin-bound and water-soluble substances in patients with acute and chronic liver failure, is a clinical application for albumin, based on its capacity to remove water-insoluble substances [37]. The MARS system has been shown to be very effective in the treatment of hepatic encephalopathy [38] and intractable pruritus. Furthermore, it markedly improves circulatory and renal functions in patients with cirrhosis and ascites [39,40]. Several randomized controlled trials have been performed to assess the use of MARS in patients with acute and chronic liver failure. Despite encouraging results, the role of MARS in the management of patients with end-stage cirrhosis remains unsettled because all reports published to date were based on small studies. Furthermore, MARS is an expensive treatment that requires skilled personnel. The use of MARS in patients with acute or chronic liver failure showed an improvement in hepatic encephalopathy, but not in systemic hemodynamic parameters or renal function [41]. Future studies will need to define the indications for MARS treatment, patient selection, and predictors for response.
Other complications of cirrhosis
Hyponatremia is common complication in patients with advanced cirrhosis. Hyponatremia is usually the result of vasopressin overactivity in response to a reduction in the effective arterial blood volume. As albumin is capable of refilling the effective arterial blood volume, albumin infusions have been used in the treatment of this complication [42]. To date, however, no study has compared the efficacy of albumin infusions with those of other volume expanders in the treatment of hyponatremia. Diuretic-induced electrolyte abnormalities can also lead to the development of hepatic encephalopathy in patients with cirrhosis. In a study conducted in cirrhotic patients with diuretic-induced hepatic encephalopathy, the infusion of albumin was comparably effective to the infusion of a colloid solution. These infusions caused similar reductions in plasma ammonia concentrations and increases in urinary ammonia excretion. The improvement of hepatic encephalopathy grade, although initially observed in both groups of patients, was only sustained at 72 hours in the group who received albumin infusions [43].
CONCLUSION
The use of albumin in patients with liver diseases has followed a seesaw evolution. Initially, it was widely used to increase serum albumin concentration and to treat ascites. At this moment, with several randomized trials and pilot studies indicating that albumin is extremely effective in the prevention and treatment of circulatory dysfunction and HRS in patients with cirrhosis, this molecule is again becoming an essential treatment in clinical hepatology. Finally, the MARS system, which has opened the hemodialysis world to patients with acute and chronic liver failure, will increase our knowledge of the mechanisms of action of albumin and will probably expand the therapeutic indications of this molecule. Without doubt, albumin will be an exciting topic for research in the near future, as it was many years ago. Future efforts should concentrate on establishing when, how much, and for what indications albumin should be used.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





