 |
 |
| Korean J Intern Med > Volume 23(4); 2008 > Article |
|
Abstract
Background/Aims
Vascular access dysfunction is an important cause of morbidity and mortality in hemodialysis (HD) patients. Recent studies have shown that a klotho gene mutation is related to endothelial dysfunction, thrombosis, and arteriosclerosis, which are regarded as causes of vascular access dysfunction. We investigated the relationship between the klotho G-395A polymorphism and early dysfunction in vascular access in HD patients.
Methods
Patients who underwent vascular access operations between 1999 and 2002 were enrolled (n=126). Genotyping was performed by allelic discrimination using a 5'-nuclease polymerase chain reaction assay. Clinical data that could be relevant to access dysfunction were obtained from medical records. Early dysfunction of vascular access was defined as the need for any angioplastic or surgical intervention to correct or replace a poorly or nonfunctioning vascular access within 1 year and at least 8 weeks after initial access placement.
Results
Of the 126 patients, the genotype frequency of G-395A was 72.2% for GG (n=91), 24.6% for GA (n=31), and 3.2% for AA (n=4), and the frequency of minor allele was 0.155. Clinical data were similar between the two groups, divided according to the status of the A allele. Early dysfunction occurred in 34 (27.0%) of patients, but it occurred at a significantly higher rate in A allele carriers (45.7%, 16/35) than in noncarriers (19.8%, 18/91; p=0.003).
Vascular access dysfunction, causing considerable morbidity and mortality, is one of the most important complications in hemodialysis (HD) patients1-4). In addition, this dysfunction is common; it occurs in up to 30% of patients in the early period following access placement operations4-7). The major pathophysiologic mechanism is venous thrombosis, following stenosis near the anastomosis site5-7). Several studies have reported that vascular access dysfunction is related to clinical factors (diabetes, old age), biochemical factors (abnormalities of cytokines, cholesterol, apolipoproteins, hemostasis-derived factors), and vascular factors (vessel size, decreased blood flow rate, intimal hyperplasia, atherosclerosis)8-12). In addition, some studies have shown that point mutations in proteins involved in the coagulation system and proinflammatory cytokines associated with endothelial dysfunction and vessel wall proliferation are related to thrombosis and atherosclerosis9-12).
The overall biological effect of the klotho gene is suppression of the aging process; mice that over-express the klotho gene have an increased life span13). Kuro-o et al.14) reported that in an experimental mouse model, klotho gene defects induced premature aging processes, including osteoporosis, infertility, senile atrophy of the gonads, thymus, and skin, physical inactivity, and pulmonary emphysema. They also suggested its involvement in progressive vascular atherosclerosis and vascular calcification14). Other studies in humans have demonstrated that the functional variant of the klotho gene (KLVS) is associated with longevity and early-onset occult coronary artery diseases (CADs)15, 16). This variant of the klotho gene has been shown to be associated with high-density lipoprotein (HDL) cholesterol, systolic blood pressure, and stroke, suggesting an association of this genetic variation with vascular atherosclerosis15). In a Korean population, Rhee et al17) showed that some klotho gene polymorphisms were related to coronary artery disease and hypertension. Kim et al18) also reported that klotho gene polymorphisms were risk factors for ischemic stroke. Notably, the klotho gene G-395A polymorphism was shown to be related to these atherosclerotic diseases in both studies. The present study was conducted to investigate the relationship between the klotho G-395A polymorphism and early vascular access dysfunction in Korean HD patients.
Between January 1999 and December 2002, 126 consecutive patients who underwent vascular access creation surgery by an experienced surgeon at our hospital were placed on maintenance HD at our hospital and an affiliated clinic. All were enrolled in the study. Patients with a history of previous access failure, those who had any problems with central veins on preoperative venography, and those who had access failures within the first 8 weeks after surgery were excluded.
This study was approved by the institutional ethics committee of our hospital. All participants provided written informed consent after being given a complete description of the study.
Clinical data collected from medical records included age, gender, underlying diseases, vascular disease, number of antihypertensive drugs, fasting glucose level, HbA1c, serum albumin, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, serum calcium and phosphorus, intact PTH, and the type and location of vascular access. Mean serum levels at three time points (the time at which dysfunction was detected, the day of the operation, and 2 months before surgery) were obtained from the laboratory data.
Early dysfunction of vascular access was defined as a situation requiring any angiographic or surgical intervention due to malfunctioning access within 1 year after surgery. Access failure within the first 8 weeks postsurgery was considered a failure of the surgery.
A buffy coat preparation was obtained from blood samples and refrigerated at -70Ōäā. Genomic DNA was extracted using a Takara DNA Purification kit (Takara Bio, Shiga, Japan). Genotyping of the G-395A polymorphism in exon 4 of the klotho gene was performed using an allelic discrimination assay. Briefly, a probe was annealed to its target sequence and the substrate generated was cleaved using the 5'-3' nuclease activity of Taq DNA polymerase (Takara Bio) as it extended from an upstream primer into the region of the probe. The probe was labeled with a "reporter" dye at its 5'-end and with a "quencher" dye at its 3'end. When intact, the proximity of the quencher substantially reduced the light emitted by the reporter. The TaqMan probe (Takara Bio) is designed to anneal to the target sequence between the traditional forward and reverse primers. During PCR, Taq DNA polymerase, with its 5'-3' nuclease activity, catalyzes primer extension, while the fluorogenic TaqMan probe hybridizes to a specific sequence on the DNA template, where it encounters the oncoming Taq DNA polymerase from upstream. The exonuclease activity of the Taq DNA polymerase cleaves the probe, with the release of reporter dye, resulting in an increased fluorescent signal, which is detected by the instrument. The detector used in this experiment was an ABI Prism 7200 sequence detection platform (Perkin Elmer Applied Biosystems, Foster City, CA, USA).
The reaction conditions used for the Taq thermocycler were 2 min at 50Ōäā, 10 min at 95Ōäā, 40 cycles for 15 s each, and then 1 min at 60Ōäā.
All statistical analyses were performed with SPSS for Windows (version 10.0; SPSS, Chicago, IL). All results are expressed as means┬▒standard deviation and a p value less than 0.05 was deemed to be statistically significant. We performed the chi-square test to confirm that the genetic frequency of the klotho polymorphism. The allele frequency of the klotho polymorphism was consistent with Hardy-Weinberg equilibrium. The chisquare test and the t-test were used to compare clinical characteristics according to klotho G-395A genotypes, the chi-square test was used to compare difference in the prevalence of early dysfunction of vascular access according to klotho G395A genotypes, and binary logistic regression analysis was performed to investigate any relationship between early access dysfunction and the klotho G-395A polymorphism, with adjustments for age, gender, type of vascular access, and diabetes mellitus. Survival rates of patients with early dysfunction of vascular access according to genotypes are expressed as medians and ranges, survival curves were prepared using the Kaplan-Meier method, and differences in the survival rate were analyzed using the log-rank test.
In total, 126 Korean patients were included; their mean age was 59.6┬▒11.6 years, and the ratio of men to women was 1.17:1. The most common cause of end-stage renal disease was diabetes (n=68, 54.0%). Fourteen patients had vascular diseases: coronary artery diseases (n=10, 7.9%), cerebrovascular diseases (n=3, 2.4%), and peripheral arterial diseases (n=1, 0.8%). A native arteriovenous fistula was created in most patients (n=108, 85.7%); of them, it was created in the forearm with radiocephalic anastomosis in most cases (n=96, 88.9%). All arteriovenous grafts were created using 6-mm diameter PTEF grafts on the forearm. The mean systolic and diastolic blood pressures were 145.31┬▒18.36 and 84.45┬▒12.79 mm Hg, respectively. The number of antihypertensive drugs being taken was 3.06┬▒1.64. The results of laboratory tests are shown in Table 1.
Of the 126 subjects, 91 (72.2%) had the GG genotype, 31 (24.6%) the GA genotype, and 4 (3.2%) the AA genotype (Table 1). The allele frequency was 0.845 for allele G and 0.155 for allele A, which was in compliance with Hardy-Weinberg equilibrium (p=0.310).
According to the A allele status, patients were divided into A allele carriers (GA+AA) and noncarriers (GG). No statistically significant differences in clinical and laboratory results were found between the groups (Table 1).
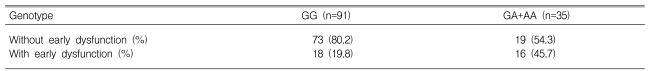
Early access dysfunction occurred in 34 patients (27.0%). According to the genotypes, this dysfunction developed in 18 (19.8%) of the patients with the GG genotype, 13 (19.8%) of those with GA, and 3 (75.0%) of those with AA. The rate of early vascular dysfunction was higher in A allele carriers than in noncarriers (45.7% vs. 19.8%, p=0.003; Table 2). This difference was statistically significant in the binary logistic regression analysis after adjusting for age, gender, type of vascular access, and diabetes mellitus (p=0.011, odds ratio=3.331; Table 3). Unexpectedly, the rate of early dysfunction was higher in male patients (p=0.016, odds ratio=3.306; Table 3).
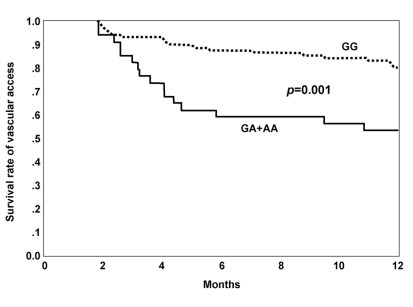
The median survival rate of patients with failed vascular access was 4.03 months (range: 1.84-11.84 months). The median survival rates of A allele carriers and noncarriers were 4.03 months (range: 1.84-10.78) and 4.65 months (range: 1.91-9.93), respectively. The survival rate of failed vascular access analyzed by the log-rank test was lower in A allele carriers than in noncarriers (p=0.001; Figure 1).
The results of this study indicated that the klotho G-395A polymorphism was related to early vascular access dysfunction in Korean HD patients. Early dysfunction of vascular access occurred in 27.0% of the patients; dysfunction was more common and occurred earlier in A allele carriers than in noncarriers. The A allele frequency of the G-395A polymorphism in the promoter of the klotho gene was 0.155, approximately midway between the values quoted from the Japanese (0.143) and Korean literature (0.170). These values in Asian populations are lower than those reported in Caucasian populations (0.196)17, 19). Previous genetic studies on vascular access failure have documented that several polymorphisms involved in the coagulation system, endothelial dysfunction, and vessel wall proliferation are related to thrombosis and endothelial dysfunction of vascular access10, 12).
Since a novel mouse mutant, klotho, was discovered incidentally by Kuro-o et al.14) many studies have been performed to uncover the secret of the aging process because the gene defect in mice leads to phenotypes resembling human premature aging processes, such as a short life span, growth retardation, senile atrophy of the genitals, thymus, and skin, defective hearing, osteoporosis, and pulmonary emphysema. Notably, mutant mice show impressive features of atherosclerosis, including medial calcification and intimal hyperplasia throughout the vasculature14). Additionally, some studies have reported that this gene influences the development of atherosclerosis through decreased endothelial NO production in mutant mice or reduced expression of the klotho gene in the kidney in hypertensive, nephrectomized, and OLETF (Otsuka Long-Evans Tokushima Fatty) rats20). Furthermore, Saito et al21) suggested that the klotho gene regulates endothelial function and atherosclerosis, based on the finding that klotho gene transfer increased NO production and prevented perivascular fibrosis in OLETF rats, an experimental animal model of atherosclerosis. Another report noted that plasminogen activator inhibitor-1, a factor for the development of thrombosis, is highly expressed in a klotho-deficient mouse22). Some studies inhumans have demonstrated that a functional variant of the klotho gene, KL-VS, is related to high-density lipoprotein (HDL) cholesterol, systolic blood pressure, longevity, coronary artery disease, and their risk factors15, 16). Based on these results, functional defects in the klotho gene may affect vascular access dysfunction caused by thrombosis and stenosis. Kawano et al19) showed that the G395A polymorphism is common and has functional relevance in a Japanese population. Additionally, two previous studies in Korean populations have suggested that this polymorphism can affect vascular diseases17, 18). In this study, the rate of early dysfunction of vascular access was higher in A allele carriers than in noncarriers (p=0.003; Table 2), and this difference was consistent with that of other important variables in the binary logistic regression analysis (p=0.011, odds ratio=3.331; Table 3). This result is an important finding in the G-395A polymorphism's involvement in vascular disease. Another key finding is that A allele carriers suffered access dysfunction earlier than noncarriers. This suggests that this polymorphism could be used to detect patients with a higher risk of early dysfunction. Further studies are warranted on this point.
Failure of vascular access in HD patients is very common and clinically important1, 2). Previous studies have shown that early dysfunction of vascular access ranged from 20% to 30%, although the definition of 'early' varied from 3 to 12 months after the operation4-7). In this study, 34 patients (27.0%) suffered early dysfunction of vascular access, defined as a situation requiring any angiographic or surgical intervention for malfunctioning access within 1 year10). The patients in this study were older (59.6 years old) than those in the two previous similar studies (49.2 and 53.0 years old) in Korea, but other clinical characteristics were similar6, 7). The proportion of males tended to be higher in A allele carriers than in noncarriers, but the difference was not significant p=0.101; Table 1).
While many studies5-9) have reported no association between gender and vascular complications, some have stated that female gender appears to be a risk factor for early failure23-25). In this study, unexpectedly, early dysfunction was associated with male gender (p=0.016, odds ratio=3.306; Table 3). Differences in vessel diameter have been suggested to account for an increase in access complications in female patients. However, as preoperative measurements of vessel diameter were made by venography in all patients at our center, adverse effects of vessel diameter can be avoided. Thus, differences in access placement practice between the United States and Korea seem to limit the extent to which results can be generalized for the Korean population. A second explanation for our finding is that male patients had more A alleles, the most powerful risk factor in our study. Moreover, the effects of the klotho gene on osteoporosis19), nonverbal reasoning26) and cardioembolic strokes18) in human diseases have been suggested to be altered by female hormones27). However, these suggestions have not been further examined or confirmed. Finally, the number of antihypertensive drugs being taken was higher in males than females (3.41┬▒1.61 vs. 2.62┬▒1.54; p=0.006), although the differences in systolic (146.43┬▒17.54 vs. 143.93┬▒19.48 mm Hg; p=0.451) and diastolic (83.87┬▒13.09 vs. 85.38┬▒12.46 mm Hg, p=0.511) blood pressures according to gender were nonsignificant. These findings suggest that a greater atherosclerotic load may occur in the vasculature of male patients compared to females, although other specific tests for load were not performed. Additionally, no difference in clinical or laboratory results was found between the two groups, divided according to the presence of early access dysfunction, in this study.
The primary limitations of our study were its small sample size and retrospective nature. The small sample size may have contributed to the difference in the ratio of males to females between A allele carriers and noncarriers (1.92:1 vs. 0.98:1, p=0.101), although the same ratio was reported in a previous study with a larger sample size21). The retrospective nature of this study may have prevented the collection of important clinical data, such as specific tests for atherosclerotic load, showing vessel wall stiffness, anatomical changes in blood flow, and vessel size, several biochemical factors (anticardiolipin antibody, lipoprotein (a), plasminogen activator inhibitor, monocyte chemoattractant-1), and history of smoking, which could affect the number of antihypertensive drugs being taken. Future prospective studies with larger sample sizes are needed to further evaluate the effect of klotho gene polymorphisms on early access dysfunction.
In conclusion, the G-395A polymorphism in the promoter region of the klotho gene may be associated with the early dysfunction of vascular access in HD patients. In particular, A allele carriers may encounter vascular access dysfunction earlier than noncarriers. This study provides the basis for clarifying the role of the klotho gene in the pathogenesis of vascular diseases.
Notes
This study was supported in part by grant no. 01-2004-21 from the Hallym University Medical Center Research Fund, and in part by a grant from the Hallym University Kidney Research Institute.
References
1. Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol 1996. 7:523ŌĆō535PMID : 8724885.


2. Woods JD, Port FK. The impact of vascular access for haemodialysis on patient morbidity and mortality. Nephrol Dial Transplant 1997. 12:657ŌĆō659PMID : 9140989.


3. Zibari GB, Rohr MS, Landreneau MD, Bridges RM, DeVault GA, Petty FH, Costley KJ, Brown ST, McDonald JC. Complication from permanent hemodialysis vascular access. Surgery 1988. 104:681ŌĆō686PMID : 3175866.

4. Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis: patency rates and results of revision. Ann Surg 1985. 202:235ŌĆō239PMID : 4015229.



5. Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 1996. 12:207ŌĆō213PMID : 8760984.


6. Moon SY, Park BJ, Lee HI, Jeong KH, Kim JH, Lee SH, Lee TW, Ihm CG, Kim MJ. Risk factors for early access failure of arteriovonous fistula in hemodialysis patients. Korean J Nephrol 2006. 25:423ŌĆō429.
7. Kim YO, Yoon SA, Kim YS, Kim MK, Oh HJ, Ku YM, Song HH, Kim KT, Chang YS, Bang BK. Early dysfunction of radiocephalic arterioveonus fistula: primary and secondary patency rate of percutaneous angioplasty. Korean J Nephrol 2003. 22:414ŌĆō419.
8. Kim YO, Yang CW, Yoon SA, Chun KA, Kim NI, Park JS, Kim BS, Kim YS, Chang YS, Bang BK. Access blood flow as a predictor of early failures of native arteriovenous fistulas in hemodialysis patients. Am J Nephrol 2001. 21:221ŌĆō225PMID : 11423692.


9. De Marchi S, Falleti E, Giacomello R, Stel G, Cecchin E, Sepiacci G, Bortolotti N, Zanello F, Gonano F, Bartoli E. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol 1996. 7:1169ŌĆō1177PMID : 8866409.


10. Girndt M, Heine GH, Ulrich C, Kohler H. Gene polymorphism association studies in dialysis: vascular access. Semin Dial 2007. 20:63ŌĆō67PMID : 17244124.


11. Kim YO, Song HC, Yoon SA, Kim YS, Yang CW, Lee SH, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. The impact of ischemic heart disease on early arteriovenous fistula failure in nondiabetic hemodialysis patients. Korean J Nephrol 2004. 23:101ŌĆō107.
12. Kim YO. Hemodialysis vascular accss dysfunction: a cellular and molecular viewpoint. Korean J Nephrol 2006. 25(Suppl):S559ŌĆōS562.

13. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science 2005. 309:1829ŌĆō1833PMID : 16123266.

14. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997. 390:45ŌĆō51PMID : 9363890.


15. Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 2005. 96:412ŌĆō418PMID : 15677572.


16. Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. klotho allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 2003. 72:1154ŌĆō1161PMID : 12669274.



17. Rhee EJ, Kim SY, Jung CH, Kim BJ, Sung KC, Kim BS, Lee WY, Kang JH, Oh KW, Lee MH, Kim SW, Park JR. The association of klotho gene polymorphism with coronary artery disease in Korean subjects. Korean J Med 2006. 70:268ŌĆō276.
18. Kim Y, Kim JH, Nam YJ, Kong M, Kim YJ, Yu KH, Lee BC, Lee C. klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett 2006. 407:189ŌĆō194PMID : 16973281.


19. Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H, Inoue S, Nabeshima Y, Nakamura K, Kuro-o M, Kawaguchi H. klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res 2002. 17:1744ŌĆō1751PMID : 12369777.


20. Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuro-o M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci 2000. 57:738ŌĆō746PMID : 10892340.


21. Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 2000. 276:767ŌĆō772PMID : 11027545.


22. Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, "klotho" mouse. Semin Thromb Hemost 2002. 28:545ŌĆō554PMID : 12536348.


23. Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ. Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis 2000. 36:1126ŌĆō1134PMID : 11096036.


24. Ernandez T, Saudan P, Berney T, Merminod T, Bednarkiewicz M, Martin PY. Risk factors for early failure of native arteriovenous fistulas. Nephron Clin Pract 2005. 101:C39ŌĆōC44PMID : 15886495.


25. De Marchi S, Falleti E, Giacomello R, Stel G, Cecchin E, Sepiacci G, Bortolotti N, Zanello F, Gonano F, Bartoli E. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol 1996. 7:1169ŌĆō1177PMID : 8866409.


Figure┬Ā1
Kaplan-Meier survival curves of vascular access according to genotypes (GG vs. GA+AA) show that the survival rate of vascular access of A allele carriers was significantly lower than that of noncarriers (p=0.001).
Continuous line: GA+AA (A allele carrier), Dashed line: GG (noncarrier).
Table┬Ā1
Comparison of general characteristics according to G395A polymorphism genotypes
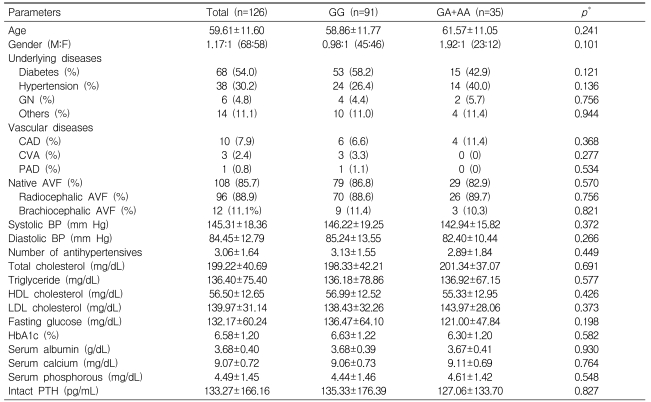
*Significantly different between patients with GG and those with GA+AA, as measured by the independent samples t-test for continuous variables and by the chi-square test for dichotomous variables.
AVF, arteriovenous fistula; BP, blood pressure; CAD, coronary arterial disease; CVA, cerebrovascular accident; GN, glomerulonephritis; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PAD, peripheral arterial disease; PTH, parathyroid hormone.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



