 |
 |
| Korean J Intern Med > Volume 20(3); 2005 > Article |
|
Abstract
Background
Cyclooxygenase (COX)-2 is the rate-limiting enzyme in prostaglandin synthesis. An increased expression has been implicated in the development and progression of human gastric cancers and colorectal adenomas and cancers. This study aimed to determine the involvement and association of COX-2 and Bcl-2 in precancerous gastric adenomas.
Methods
Seventy-nine gastric polyps were obtained by endoscopic mucosal resection or polypectomy from January, 2000 to July, 2003. Immunohistochemical expression of COX-2 and Bcl-2 was observed, and their relationships with various clinicopathological factors were analyzed.
Results
Histologically, 13 hyperplastic polyps and 66 tubular adenomas, of which 17 showed high-grade dysplasia, were observed. Increased COX-2 expression was observed in low-grade and high-grade tubular adenomas compared to hyperplastic polyps (p=0.004 and p=0.001, respectively). COX-2 expression was significantly higher in larger (>1 cm) compared with smaller (Ōēż1 cm) tubular adenomas (p=0.034), but no relation was observed in hyperplastic polyps. While Bcl-2 expression differed significantly according to histology, increased Bcl-2 expression was observed especially in COX-2 positive low-grade tubular adenomas.
Gastric cancer is still one of the major causes of cancer death in the world, although its incidence is decreasing. Gastric adenomas, a relatively common benign neoplasm of the glandular epithelium, are widely considered to be precursor lesions with an increased risk of malignancy1, 2). While a correlation between the colonic adenoma-carcinoma sequence and multi-step genetic alterations has been reported, the exact pathways of gastric carcinogenesis are still unknown, though a large number of genetic alterations have been found to regulate the initiation and progression of gastric cancer3).
Epidemiologic studies have shown that the use of aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) are associated with a reduced risk of colorectal, breast, esophageal, gastric and lung cancer4, 5). Furthermore, clinical trials have shown that NSAIDs cause regression of colorectal adenomatous polyps in patients with familial adenomatous polyposis (FAP)6, 7). Increased COX-2 expression has been reported in colorectal adenomas and adenocarcinomas8-11). Recently, COX-2 expression has been reported in human gastric adenomas and adenocarcinomas, suggesting that COX-2 plays an important role in gastric tumorigenesis as well12-19).
Several mechanisms by which COX-2 may contribute to tumorigenesis have been suggested20). Increased COX-2 expression has been shown to inhibit apoptosis and may be modulated by Bcl-2 expression21). Increased COX-2 expression may also promote angiogenesis10, 22), enhance cell motility and adhesion23), and inhibit immune surveillance20).
The aim of this study was first, to examine the relationship between COX-2 and Bcl-2 expression and clinical characteristics in precancerous gastric adenomas and second, to determine the correlation of COX-2 and Bcl-2 expression in gastric adenomas.
Seventy-nine gastric polyps were obtained from patients that received an endoscopic mucosal resection or polypectomy from January, 2000 to July, 2003 at Kyungpook National University Hospital. Patient characteristics were obtained from medical records. The patient pool consisted of 52 males and 27 females. The mean age was 62.91┬▒8.92 years. No patients had been treated with any non-steroidal anti-inflammatory drugs (NSAIDs) or COX-2 inhibitors. Histologically, there were 13 hyperplastic polyps and 66 tubular adenomas, of which 17 showed high grade dysplasia. In addition, gastric mucosa specimens from five healthy volunteers were examined as normal controls.
The specimens were fixed in 10% buffered formalin and processed into paraffin blocks according to established protocols. All hematoxylin and eosin (H&E) stained slides for each specimen were reviewed and a slide displaying a characteristic finding was selected from each case. Using a tissue microarray, a tissue cylinder with a diameter of 2 mm was punched through the selected tumor areas from each "donor" tissue block. Tissue cylinders were then inserted into "recipient" tissue array agarose blocks. Finally, the agarose blocks were embedded into paraffin. Sections of the recipient paraffin block were cut at 5 ┬Ąm, and dried in a 60Ōäā oven for 2 to 4 hours.
The tissue sections were deparaffinized with xylene and rehydrated with grading ethanol. Antigen retrieval was performed with a 0.01M, pH 6.0 citrate buffer in a pressure cooker for 10 minutes, and the slides were then cooled down to room temperature. The slides were incubated with 3% H2O2 in methanol for 10 minutes to block endogenous peroxidase activity. Next, the specimens were blocked with a blocking solution (Zymed, San Francisco, California, USA) for 30 minutes and incubated overnight at 4Ōäā with the primary antibody Cox-2 (1:100, Transduction Laboratories, Lexington, Kentucky, USA) and Bcl-2 (1:30, Zymed, San Francisco, California, USA). The specimens were incubated with a secondary antibody (Zymed, San Francisco, California, USA) for 30 minutes following multiple rinses. After rinsing with PBS three times, excess liquid was gently tapped off and streptoavidin peroxidase conjugate (Zymed, San Francisco, California, USA) was applied for 30 minutes. A chromogen reaction was performed with diaminobenzidine (Zymed, South San Francisco, California, USA) for 10-20 minutes, which produced a strong brown color for positive staining. Sections were counterstained with Mayer's hematoxylin for 30 seconds. Stained slides were dehydrated and cleared with grading ethanol, xylene. Finally, the slides were mounted with malinol.
The immunostaining was evaluated by two independent observers. COX-2 immunostaining was graded on a scale of 5 based on the intensity and extent of perinuclear and cytoplasmic staining of the gastric epithelial cells: 0=no staining, 1=weak or moderate intensity in less than 10% of the tumor area, 2=weak or moderate intensity in 10~50% of the tumor or strong intensity in less than 10% of the tumor area, 3=weak or moderate intensity in the majority of the tumor or strong intensity in 10~50% of the tumor area and 4=strong intensity in the majority of the tumor area. Specimens with a grade of more than 2 were regarded as having a positive expression.
Bcl-2 immunostaining was regarded as positive when expressing a cytoplasmic or perinuclear staining of more than 50% of the tumor cells.
The Žć2 test and Fisher's exact probability test, where appropriate, were used to compare the expression of COX-2 and Bcl-2 with various clinicopathological parameters. The relationship between COX-2 and Bcl-2 were analyzed by the Pearson correlation coefficient. All statistical analyses were performed by SPSS (version 11.0 SPSS Inc., IL, USA) and p values <0.05 were considered to be statistically significant.
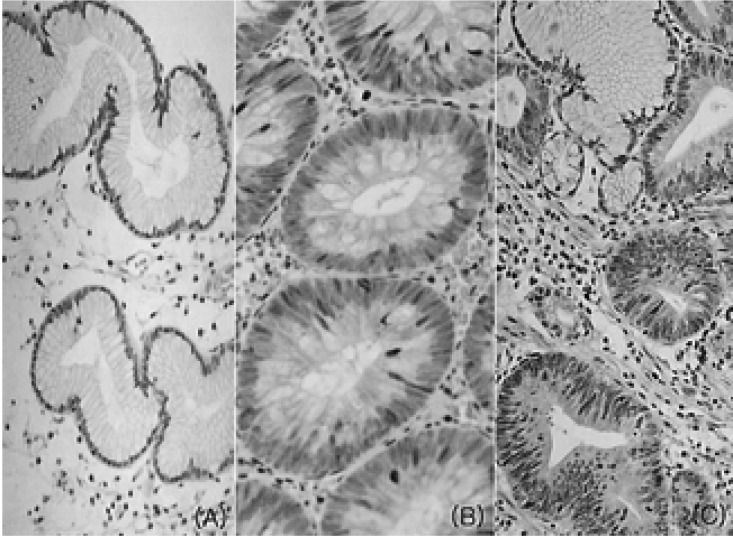
In normal gastric mucosa, no COX-2 immunoreactivity was observed in gastric epithelial cells, but fibroblasts and inflammatory cells located mainly in the upper lamina propria were immunoreactive. In hyperplastic polyps, COX-2 immunoreactivity was rarely observed in gastric epithelial cells (Figure 1A). In tubular adenomas, COX-2 immunoreactivity was observed not only in fibroblasts and inflammatory cells but also in dysplastic gastric epithelial cells (Figure 1B, 1C).
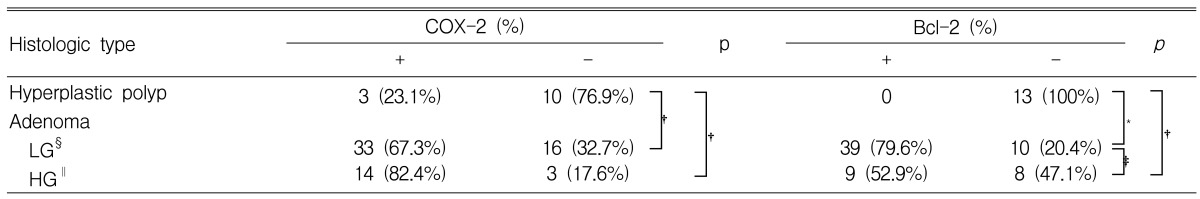
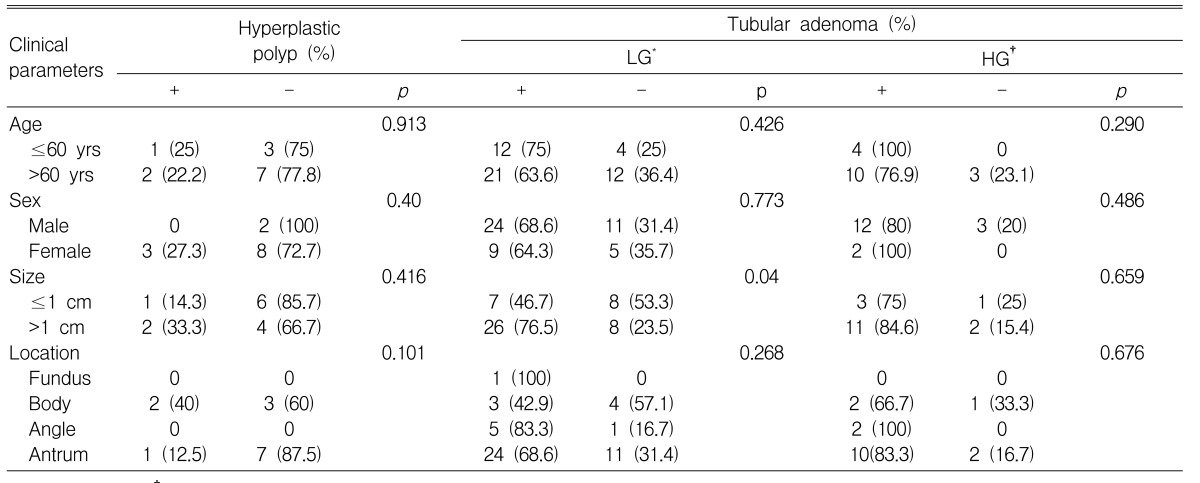
Positive expression of COX-2 was observed in 3 of 13 (23.1%) hyperplastic polyps, 33 of 49 (67.3%) low-grade tubular adenomas and 14 of 17 (82.4%) high-grade tubular adenomas (Table 2). Increased COX-2 expression was observed in low-grade and high-grade tubular adenomas compared to hyperplastic polyps (p=0.004 and p=0.001, respectively).
COX-2 expression significantly increased in tubular adenomas >1 cm compared to tubular adenomas Ōēż1 cm (p=0.034). This difference was especially prominent in low-grade tubular adenomas, which displayed a positive COX-2 expression in 7 of 15 (46.7%) adenomas Ōēż1 cm and 26 of 34 (76.5%) adenomas >1 cm (p=0.04). There was no correlation between COX-2 expression and the size of hyperplastic polyps.
COX-2 expression was not associated with age, sex or location in both hyperplastic polyps and tubular adenomas.
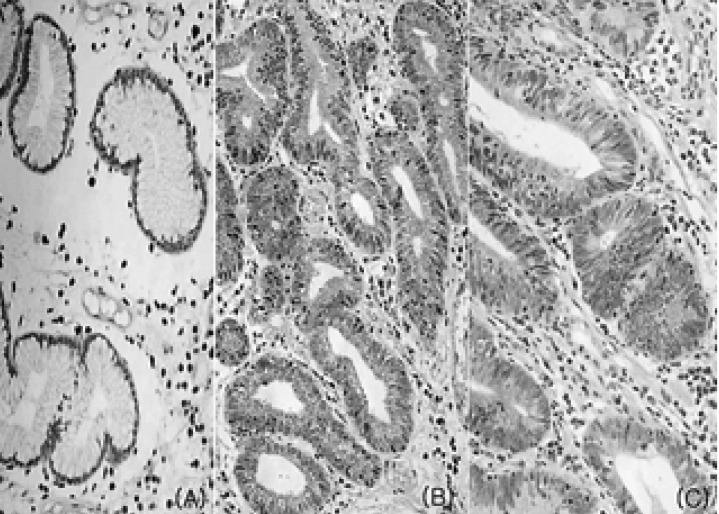
In normal gastric mucosa and hyperplastic polyps, Bcl-2 immunoreactivity was limited to minimal staining in the gastric epithelial regenerative compartments, the intestinal crypt bases and the gastric mucous neck region and strong staining in lymphocytes in the lamina propria (Figure 2A). In tubular adenomas, Bcl-2 immunoreactivity was observed in a diffuse cytoplasmic or focal nuclear pattern in most gastric epithelial cells (Figure 2B, 2C).
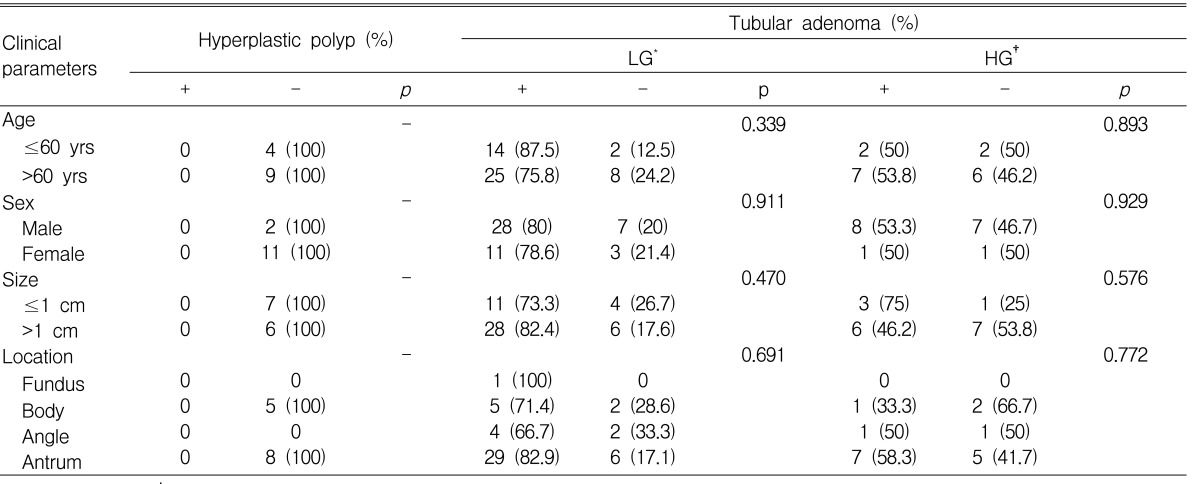
Positive expression of Bcl-2 was not observed in any of the 13 (0%) hyperplastic polyps, 39 of 49 (79.6%) low-grade tubular adenomas and 9 of 17 (52.9%) high-grade tubular adenomas (Table 2). Bcl-2 expression differed significantly according to histology (p<0.001).
There was no statistically significant relationship between Bcl-2 expression and age, sex, location or size of both hyperplastic polyps and tubular adenomas.
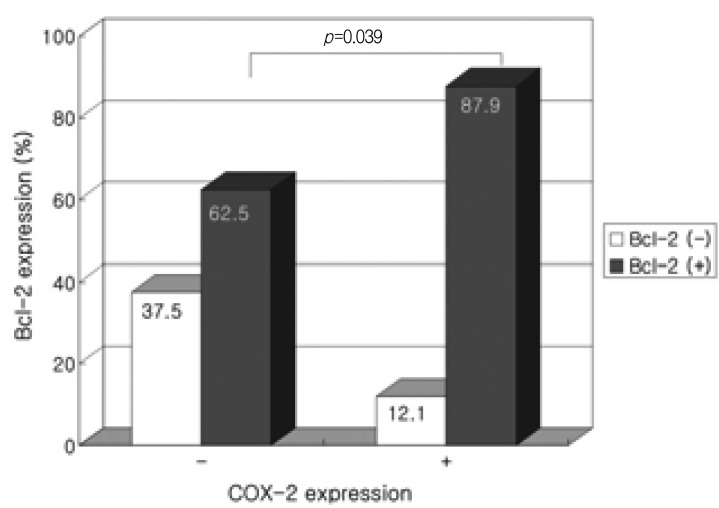
In low-grade tubular adenomas, positive Bcl-2 expression was observed in 29 of 33 (87.9%) COX-2 positive tubular adenomas compared to 10 of 16 (62.5%) COX-2 negative adenomas, showing a significantly increased Bcl-2 expression in COX-2 positive tubular adenomas (p=0.039). Though not statistically significant, a tendency of increased Bcl-2 expression in COX-2 positive high-grade tubular adenomas was observed (p=0.072).
Cyclooxygenase (COX) is the rate-limiting enzyme in prostaglandin (PG) synthesis and two isoforms have been identified24). COX-1 is constitutively expressed in most normal tissues and plays an important role in maintaining the integrity of gastrointestinal mucosa, renal function, and platelet aggregation. COX-2 is undetectable in most tissues, but is induced by various stimuli such as mitogens, cytokines and tumor promoters thus contributing to the synthesis of PGs in inflamed and malignant tissues. Increased COX-2 expression has been observed in various cancers, especially gastrointestinal cancers. Though a number of studies have reported the increased expression of COX-2 in gastric cancer, limited data are available on COX-2 expression in gastric adenomas. This study demonstrated the expression and influence of COX-2 in gastric adenomas.
In the present study, COX-2 expression increased in tubular adenomas compared to hyperplastic polyps. In addition, although not statistically significant, an increased expression of COX-2 was observed in high-grade compared to low-grade tubular adenomas. Einspahr et al.11) reported similar results to ours in a study of colorectal adenomas, where an increased expression, though not statistically significant, of COX-2 was noted in high-grade dysplasia compared with mild- to moderate-grade dysplasia, which provided contrary results to previous studies8) where the degree of dysplasia was not related to COX-2 expression. Van Rees et al.18) reported an increased expression of COX-2 in parallel with the progression of reactive epithelium to the highest degree of epithelial dysplasia in the human stomach. Our results suggest that the expression of COX-2 may be related to the progression of the neoplastic process, but further studies with a larger size of high-grade tubular adenomas are needed.
Though there are few reports on the correlation of COX-2 expression and gastric adenoma size, many reports on the size-dependent expression of COX-2 in colorectal adenomas11, 25) support the hypothesis that increased COX-2 expression provides a growth advantage for adenomas. In the present study, COX-2 expression was significantly higher in larger (>1 cm) compared to smaller (Ōēż1 cm) tubular adenomas. This difference was especially prominent in low-grade tubular adenomas. This result suggests that COX-2 expression may have a similar role in adenoma growth in both gastric adenomas and colorectal adenomas.
COX-2 expression is not only related to cell growth promotion but increased COX-2 expression has also been shown to inhibit apoptosis21). Bcl-2 is a proto-oncogene, which inhibits apoptosis and prolongs cell life span26). Bcl-2 was expressed in 65% of the cases of chronic atrophic gastritis with intestinal metaplasia, in 81% of gastric epithelial dysplasia27) and in 72% of gastric adenocarcinomas28). The incidence of Bcl-2 expression, based on cytoplasmic expression, is generally higher in both colonic29-31) and gastric adenomas32-34) than carcinomas29-34), implicating a potential role for Bcl-2 in the early phase of the adenoma-carcinoma sequence. In the present study, Bcl-2 expression differed according to histology and was significantly higher in low-grade tubular adenomas compared to high-grade tubular adenomas. While various results have been reported on the relationship between Bcl-2 expression and the degree of dysplasia27, 35), our results were compatible with that of Uesugi et al.35) who also reported an increased expression of Bcl-2 in mild compared to severe dysplasia. Bcl-2 overexpression has been suggested to prevent apoptosis in adenomas leading to extended survival and prolonged exposure of proliferative cells to carcinogenic stimuli, increasing the chance of mutations progressing to carcinomas. Afterwards, other genes, such as p53, may dominate the process and suppress the expression of Bcl-2 in carcinomas. The increased expression of Bcl-2 in low-grade compared to high-grade tubular adenomas in this study supports the hypothesis of a potential role for Bcl-2 in the early stage of adenoma progression.
Accumulating evidence indicates that induction of COX-2 correlates with increased Bcl-2 expression. Sheng et al.21) demonstrated that PGE2 inhibited apoptosis and induced Bcl-2 expression in human colon cancer cells, suggesting that apoptosis inhibition by COX-2 expression may be modulated by Bcl-2 expression. The increased expression of Bcl-2 especially in COX-2 positive low-grade tubular adenomas seen in this study suggests that this hypothesis may be applicable in gastric adenomas as well, especially in the early stages of adenoma formation.
The above results suggest that overexpression of COX-2 constitutes an early event in gastric tumorigenesis. NSAIDs and COX-2 inhibitors have been shown to reduce polyp burden in patients with FAP6, 7). Recently, Saukkonen et al.36) showed that inhibition of COX-2 with a COX-2 inhibitor, celecoxib, disturbed the integrity of gastric adenomas. These findings support further studies on the possible role of COX-2 inhibitors as a chemotherapeutic modality against gastric tumorigenesis.
In summary, increased COX-2 expression was observed in precancerous tubular adenomas and COX-2 expression increased in a size-dependent manner in tubular adenomas, suggesting a role in polyp growth. The increased expression of Bcl-2 in tubular adenomas, especially in COX-2 positive low-grade tubular adenomas, suggests that Bcl-2 may contribute to the adenoma-carcinoma sequence in the early phase and that COX-2 action may be related to Bcl-2 expression.
References
1. Antonioli DA. Precursors of gastric carcinoma: a critical review with a brief description of early (curable) gastric cancer. Hum Pathol 1994;25:994ŌĆō1005PMID : 7927322.


2. Oberhuber G, Stolte M. Gastric polyps: an update of their pathology and biological significance. Virchows Arch 2000;437:581ŌĆō590PMID : 11193468.


3. Tahara E, Semba S, Tahara H. Molecular biological observations in gastric cancer. Semin Oncol 1996;23:307ŌĆō315PMID : 8658214.

4. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991;325:1593ŌĆō1596PMID : 1669840.


5. Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr. Aspirin use and risk of fatal cancer. Cancer Res 1993;53:1322ŌĆō1327PMID : 8443812.

6. Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993;328:1313ŌĆō1316PMID : 8385741.


7. Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in APC╬ö716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996;87:803ŌĆō809PMID : 8945508.


8. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994;107:1183ŌĆō1188PMID : 7926468.


9. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995;55:3785ŌĆō3789PMID : 7641194.

10. Cianchi F, Cortesini C, Bechi P, Fantappie O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, Masin E. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology 2001;121:1339ŌĆō1347PMID : 11729113.


11. Einspahr JG, Krouse RS, Yochim JM, Danenberg PV, Danenberg KD, Bhattacharyya AK, Martinez ME, Alberts DS. Association between cyclooxygenase expression and colorectal adenoma characteristics. Cancer Res 2003;63:3891ŌĆō3893PMID : 12873979.

12. Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997;57:1276ŌĆō1280PMID : 9102213.

13. Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res 2000;6:519ŌĆō525PMID : 10690533.

14. Saukkonen K, Nieminen O, van Rees B, Vilkki S, Harkonen M, Juhola M, Mecklin JP, Sipponen P, Ristimaki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res 2001;7:1923ŌĆō1931PMID : 11448905.

15. Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol 2001;76:26ŌĆō30PMID : 11223821.


16. Chae SW, Sohn JH, Shin HS, Park YE. Expression of cyclooxygenase-2 protein in gastric carcinogenesis. Cancer Res Treat 2002;34:252ŌĆō257.


17. Joo YE, Oh WT, Rew JS, Park CH, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion 2002;66:222ŌĆō229PMID : 12592098.


18. van Rees BP, Saukkonen K, Ristimaki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol 2002;196:171ŌĆō179PMID : 11793368.


19. Saukkonen K, Rintahaka J, Sivula A, Buskens CJ, van Rees BP, Rio MC, Haglund C, van Lanschot JJ, Offerhaus GJ, Ristimaki A. Cyclooxygenase-2 and gastric carcinogenesis. APMIS 2003;111:915ŌĆō925PMID : 14616542.


20. Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol 2002;190:279ŌĆō286PMID : 11857443.


21. Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998;58:362ŌĆō366PMID : 9443418.
22. Kawada M, Seno H, Wada M, Suzuki K, Kanda N, Kayahara T, Fukui H, Sawada M, Kajiyama T, Sakai M, Chiba T. Cyclooxygenase-2 expression and angiogenesis in gastric hyperplastic polyp: association with polyp size. Digestion 2003;67:20ŌĆō24PMID : 12743436.


23. Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem 2001;276:18075ŌĆō18081PMID : 11278548.


24. Williams CW, DuBois RN. Prostaglandin endoperoxide synthase; why two isoforms? Am J Physiol 1996;270:G393ŌĆōG400PMID : 8638704.


25. Azumaya M, Kobayashi M, Ajioka Y, Honma T, Suzuki Y, Takeuchi M, Narisawa R, Asakura H. Size-dependent expression of cyclooxygenase-2 in sporadic colorectal adenomas relative to adenomas in patients with familial adenomatous polyposis. Pathol Int 2002;52:272ŌĆō276PMID : 12031082.


26. Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990;348:334ŌĆō336PMID : 2250705.


27. Lauwers GY, Scott GV, Hendricks J. Immunohistochemical evidence of aberrant bcl-2 protein expression in gastric epithelial dysplasia. Cancer 1994;73:2900ŌĆō2904PMID : 8199986.


28. Lauwers GY, Scott GV, Karpeh MS. Immunohistochemical evalua tion of bcl-2 protein expression in gastric adenocarcinomas. Cancer 1995;75:2209ŌĆō2213PMID : 7712429.


29. Hao XP, Ilyas M, Talbot IC. Expression of Bcl-2 and p53 in the colorectal adenoma-carcinoma sequence. Pathobiology 1997;65:140ŌĆō145PMID : 9309780.


30. Saleh HA, Jackson H, Khatib G, Banerjee M. Correlation of bcl-2 oncoprotein immunohistochemical expression with proliferation index and histopathologic parameters in colorectal neoplasia. Pathol Oncol Res 1999;5:273ŌĆō279PMID : 10607921.


31. Nomura M, Watari J, Yokota K, Saitoh Y, Obara T, Kohgo Y. Morphogenesis of nonpolypoid colorectal adenomas and early carcinomas assessed by cell proliferation and apoptosis. Virchows Arch 2000;437:17ŌĆō24PMID : 10963375.


32. Kim YS, Kim BK, Shim SI. Expression of bcl-2 protein in gastric adenoma and adenocarcinoma. Korean J Pathol 1998;32:248ŌĆō254.
33. Kyokane K, Ito M, Sato Y, Ina K, Ando T, Kusugami K. Expression of Bcl-2 and p53 correlates with the morphology of gastric neoplasia. J Pathol 1998;184:382ŌĆō389PMID : 9664903.


34. Lee SS, Cho KJ, Hong SI, Myoung NK, Jang JJ. Nuclear overexpression of bcl-2 oncoprotein during the progression of human stomach cancer. J Korean Med Sci 1998;13:153ŌĆō158PMID : 9610615.



Figure┬Ā1
COX-2 immunostaining in hyperplastic polyps and tubular adenomas of the stomach. (A) In hyperplastic polyps, no immunoreactivity was observed in epithelial cells but positive staining was observed in fibroblasts and inflammatory cells located in the upper portion of the lamina propria (├Ś200). (B, C) Positive immunoreactivity was observed focally in the cytoplasm and perinuclear area of gastric epithelial cells in low-grade (B, ├Ś200) and high-grade tubular adenomas (C, ├Ś200).
Figure┬Ā2
Bcl-2 immunostaining in hyperplastic polyps and tubular adenomas of the stomach. (A) In hyperplastic polyps, focal minimal immunoreactivity was limited to epithelial regenerative compartments and strong immunoreactivity was observed in lymphocytes located in the lamina propria (├Ś200). (B) Diffuse strong positive immunoreactivity was observed in most of the gastric epithelial cells in low-grade tubular adenomas (├Ś200). (C) In high-grade tubular adenomas, positive immunoreactivity was observed in a focal nuclear and cytoplasmic pattern (├Ś200).




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



