INTRODUCTION
Strongyloides stercoralis is a soil-transmitted nematode of worldwide distribution, but a high incidence of infection is correlated with low socioeconomic areas in temperate zones1,2).
Hyperinfection risk factors are age, underlying chronic obstructive pulmonary disease, cortico steroid treatment and antacid or cimetidine treatment3,4). Purtilo et al. reported thirty-two cases of fatal strongyloidiasis in immunosuppressed patients5). In Korea, only one case of strongyloidiasis hyperinfection was reported by Choi et al. in 1985 until now1).
We experienced a case of hyperinfection with Strongyloides stercoralis in a 64-year-old housewife who had received corticosteroids for several months because of arthritis.
CASE REPORT
A 64-year-old housewife was admitted to our hospital because of epigastric pain associated with nausea, vomiting and general weakness for one month prior to admission.
In her past history, she had an appendectomy in 1986, a polypectomy for a colon polyp in 1987 and received corticosteroids for treatment of arthritis for several months prior to admission.
On physical examination, the pulse was 96/min, blood pressure was 150/100mmHg, and body temperature was 36.5┬░C by mouth. The patient appeared chronically ill with a puffy face and buffalo hump. The lungs were clear, and the heart and breasts were normal. The abdomen was distended and there was decreased bowel sound with ascites. The liver was palpable 2cm below the Rt. lower costal margin without tenderness. There was pitting edema of the lower extremities. The white-cell count was 13,700/mm3 with eosinophil 6% and hemoglobin 12.3 g/dl, hematocrit was 36%, serum glucose 77mg/dl, postprandial glucose 145mg/dl, total protein 5.8g/dl, albumin 2.6g/dl, and potassium 3.83mEq/l. Stool occult blood was strongly positive. Cortisol assay was 4.40╬╝g/dl at 8:00 A.M. and 2.05╬╝g/dl at 4:00 P.M. ACTH was 15.91pg/ml.
Chest X-ray revealed increased interstitial marking and vascular blurring on both lower lung fields and streaky fibrotic density on the Rt. middle lung field.
On the eighth hospital day, she was administrated prednisolone under the impression of iatrogenic Cushing's disease. But vomiting continued and the serum potassium level dropped to 2.96 mEq/l.
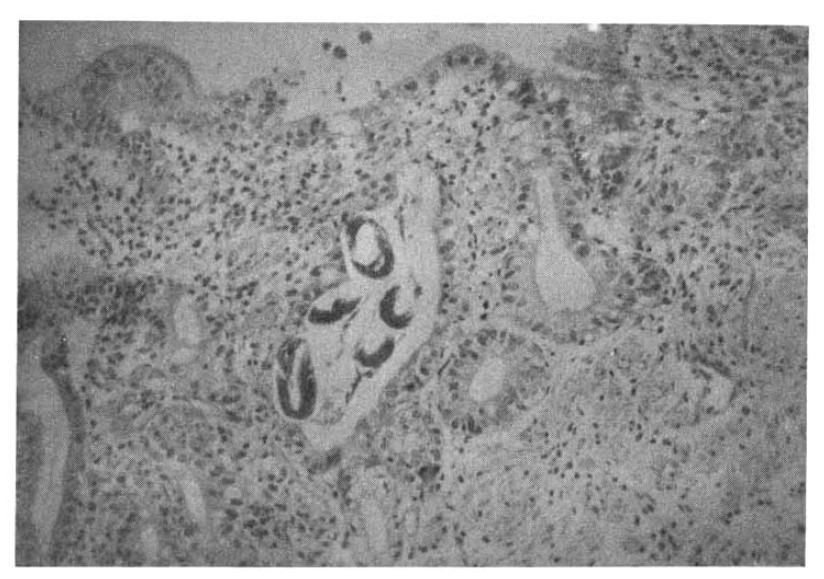
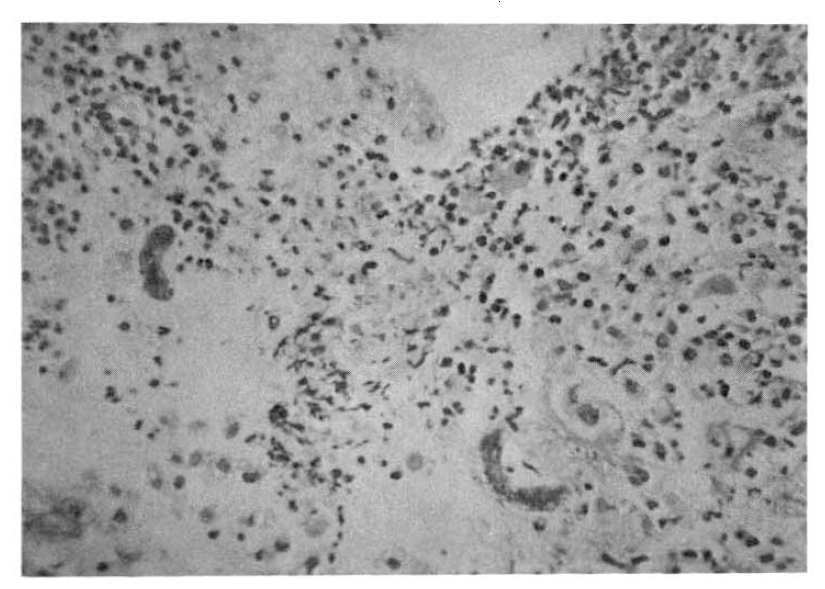
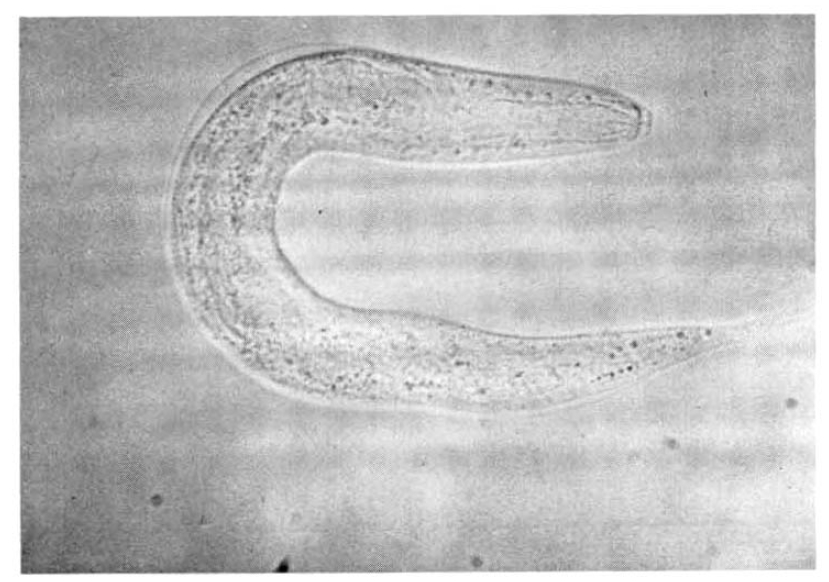
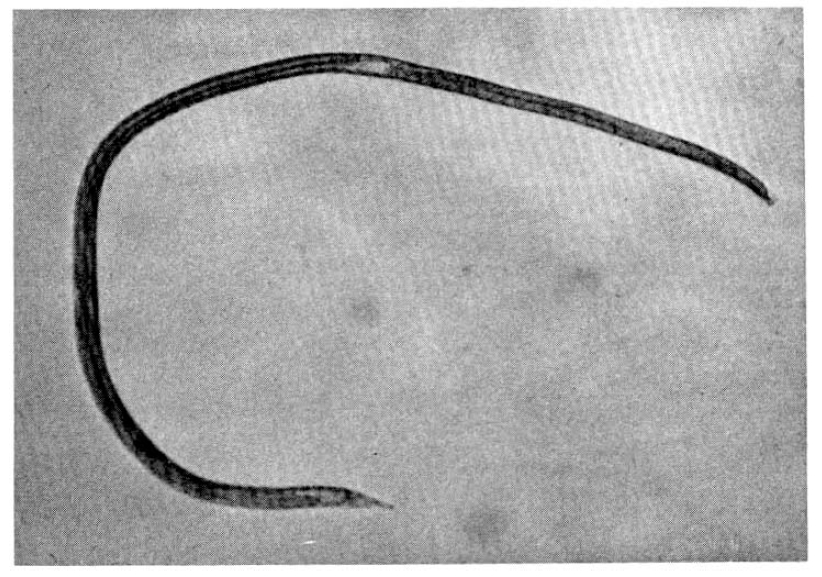
On the 18th hospital day, gastroduodenoscopy showed a mid-esophageal diverticulum and hemorrhagic gastritis (Fig. 1). Strongyloides infection confirmed by biopsy and microscopic examination showed the adult form of strongyloides stercoralis. Cortico-steroid and antacid treatment were discontinued. The repeated gastroduodenoscopy and gastroduodenal juice analysis definitely disclosed the infection with Strongyloides stercoralis on the 24th hospital day (Fig. 2, 3).
Serum albumin was 2.1 g/dl, serum potassium was 2.86 mEq/l and serum total bilirubin was 3.1mg/dl on the 31st hospital day. On the 44th hospital day, serum total bilirubin reached 10.1mg/dl, GOT was 75 IU/I, and GPT was 31 IU/I. Pressure sores and friction blisters developed on the buttock and inguinal area.
She complained of progressive cough, sputum and shortness of breath. There were rales on both lower lung fields and diminished breath sound at the base of the Rt. lung.
She took albendazole 200mg/day for 6 days from the 22nd hospital day and for 4 days from the 45th hospital day. Severe dyspnea and oliguria developed and she expired on the 79th hospital day.
DISCUSSION
Infection with the nematode Strongyloides stercoralis is potentially lethal because of its capacity to cause an overwhelming autoinfection, particularly in the immunosuppressed host4).
Strongyloides stercoralis is widely distributed in tropical and subtropical areas, where the climate is one of high temperature and high humidity2). It may exist as a free-living adult in soil developing from the rhabditiform, first-stage larvae passed in feces. Several adult generations may ensue, but progressively more of the larvae arising from the free forms molt into filariform, infective forms that invade their human host through the skin. After penetration, the larvae migrate into the circulatory system, arising in the lungs where partial maturation with fertilization may take place. Eventually the adolescent males and females migrate through the tracheobronchial tree and esophagus into the intestinal tract where the adult female worm establishes itself by burrowing into the mucosa, and the male is eliminated in the feces. Under certain conditions, hyperinfection occurs and is manifested by maturation of worms with reproduction in various parts of the body, particularly the bronchial tree. Under other specific conditions, rhabditiform larvae in the intestinal tract may have time to transform into infective larvae. Strains of varying infectivity and virulence are known to exist6).
Giannella et al. and berk et al. reported the risk factors for infection as age and use of chronic corticosteroids, H2 blockers and antacid3,4). In our case, the patient had received corticosteroid therapy for several months because of arthritis.
The clinical manifestations are asymptomatic in 50% of the cases, but in are characterized severe strongyloidiasis by a burning sensation in the epigastrium or colicky abdominal pain, watery or mucoid diarrhea, nausea, vomiting, constipation, gastro-intestinal bleeding, pruritis ani, paralytic ilues, weight loss with malabsorption or protein loosing enteropathy, hypoproteinemia, hypokalemia, skin rash and urticaria, cough, wheezing, shortness of breath, hemoptysis, meningitis and brain abscess and eosinophilia. Our patient complained of epigastric pain associated with nausea, vomiting and general weakness. Eosinophil counts in strongyloidiasis have tended to be lower with corticosteroid administration, with systemic bacterial infection, and with the hyperinfection syndrome4ŌĆō9).
A diffuse edematous catarrhal enteritis may progress, by lytic enzyme produced by the parasite and with the contribution of bacteria, to hemorrhagic enterocolitis and fibrosis. In our case, hemorrhagic gastritis was revealed on gastroduodenoscopy. The adjacent lymphatic glands and ducts may also become involved, resulting in granulomatous lymphangitis that contributes further to the congestion and edema of the gut. Thus, it is not surprising to see malabsorption as the presenting syndrome in strongyloidiasis6,10). The mucosal ulcerations produced by this parasite, together with the contributing phagedenic ulcers, may play a prominent role in the gram-negative septicemia that is occasionally seen in hyperinfection with Strongyloides stercoralis6).
Early diagnosis is essential so that specific treatment is instituted immediately. The direct method of stool examination may fail to show the larvae initially, and more reliable special culture techniques may have to be used. Stools should be repeatedly examined for ova and parasites11). In our case, stools showed an abundant adult form of strongyloides stercoralis. Gastric aspiration is essential if stools are negative. Sampling the duodenal or small intestinal contents can reveal Strongyloides stercoralis. Gastric and small intestinal biopsy has been very helpful when histologic examination of the intestinal mucosa is needed4,12,13). In our case, gastroduodenal juice analysis disclosed S. stercoralis. If pulmonary symptoms appear, the sputum should also be examined for parasites11).
Since Strongyloides stercoralis has a worldwide distribution, it is important that physicians dealing with diseases of altered immunity, such as lymphomas, leukemias, leprosy, systemic lupus erythematosus, and related disorders, become aware of this syndrome. Even though the parasite is found most commonly in the tropics, it is frequently found in the temperate climate.
Thiabendazole is the treatment of choice for strongyloidiasis14) and is quite effective in most cases. It is given in a dose of 25mg/kg twice a day for 2 days. In the hyperinfection syndrome, early diagnosis and treatment for 2ŌĆō3 weeks may be life saving, but the mortality is very high despite treatment. Patients with a past history of exposure to S. stercoralis should be thoroughly examined and treated before undergoing any immune-suppressed therapy8).
Our patient was treated with albendazole as thiabendazole was not available in our country.
G. Schad reported in 1986 that cyclosporin a has effective antiparasitic properties against Strongyloides stercoralis15).




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





